Voyager’s EMH is coming soon.



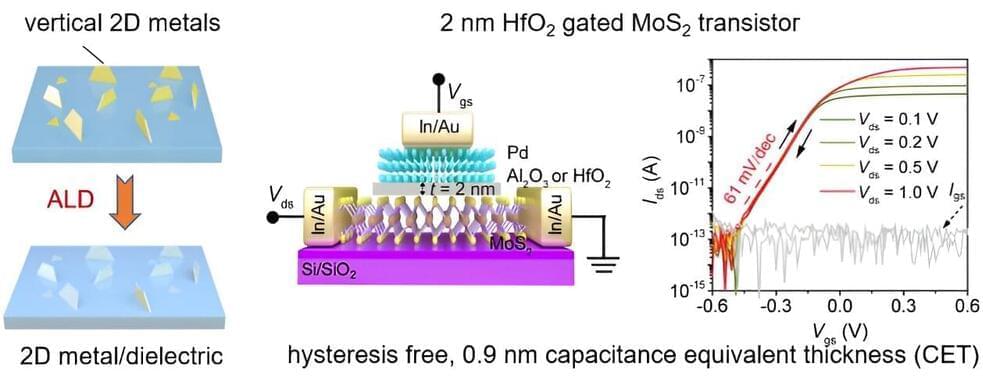
The effective integration of extremely thin insulating layers with two-dimensional (2D) semiconductors could enable the fabrication of 2D transistors with an electrical capacitance comparable to SiO2 with thicknesses below 1-nm. These transistors could, in turn, help to boost the performance and reduce the power consumption of electronic devices.
Researchers at Nankai University in China recently introduced a new strategy to synthesize single-crystalline metal nanosheets that could be easily transferred onto 2D substrates. This strategy, outlined in a paper in Nature Electronics, was successfully used to deposit 2-nm-thick dielectrics based on Al2O3 or HfO2 for highly performing top-gated transistors.
“At the very beginning, we aimed to developing the chemical vapor deposition (CVD) synthetic strategy of 2D Cu2O, which is a p-type high-mobility 2D semiconductor,” Jinxiong Wu, corresponding author of the paper, told Tech Xplore.

ACE, a groundbreaking DNA-powered signal amplification technology, significantly enhances the sensitivity of mass cytometry, providing new insights into various biological and pathological processes.
Since the 1950s, researchers have employed “flow cytometry,” a renowned technique devised by Wallace Coulter, to characterize various types of immune cells in research studies and human blood samples. This method has significantly enhanced our understanding of immune cell development and provided innovative approaches for evaluating human health and diagnosing various blood cancers. Eventually, flow cytometry was extended to analyze other cell types as well.
In traditional flow cytometry, cell surface and intracellular proteins are detected with antibody molecules that are linked to fluorescent probes. However, while providing single-cell sensitivity, this method is limited in detecting multiple proteins by the number of fluorophores that can be clearly distinguished within the entire spectrum of fluorescent light.

Summary: Researchers developed a computer model that mimics how the hippocampus stores new episodic memories without erasing old ones. This model demonstrates that the CA3 region of the hippocampus serves as an anchor point for memories, allowing efficient storage in surrounding regions.
The findings reveal insights into how the brain organizes personal experiences and maintains stability despite constant updates. The model shows promise for enhancing our understanding of memory retention and cognitive processing.
The ability to accurately quantify biological age could help monitor and control healthy aging. Epigenetic clocks have emerged as promising tools for estimating biological age, yet so far, most of these clocks have been developed from heterogeneous bulk tissues, and are thus composites of two aging processes, one reflecting the change of cell-type composition with age and another reflecting the aging of individual cell-types. There is thus a need to dissect and quantify these two components of epigenetic clocks, and to develop epigenetic clocks that can yield biological age estimates at cell-type resolution. Here we demonstrate that in blood and brain, approximately 35% of an epigenetic clock’s accuracy is driven by underlying shifts in lymphocyte and neuronal subsets, respectively. Using brain and liver tissue as prototypes, we build and validate neuron and hepatocyte specific DNA methylation clocks, and demonstrate that these cell-type specific clocks yield improved estimates of chronological age in the corresponding cell and tissue-types. We find that neuron and glia specific clocks display biological age acceleration in Alzheimer’s Disease with the effect being strongest for glia in the temporal lobe. The hepatocyte clock is found accelerated in liver under various pathological conditions. In contrast, non-cell-type specific clocks do not display biological age-acceleration, or only do so more marginally. In summary, this work highlights the importance of dissecting epigenetic clocks and quantifying biological age at cell-type resolution.
The authors have declared no competing interest.
The Illumina DNA methylation datasets analyzed here are all freely available from GEO (www.ncbi.nlm.nih.gov/geo).


NASA awarded SpaceX a contract for $843 million to make a giant version of the Dragon Spacecraft to de-orbit the Space Station. It will use 47 Draco engines instead of the 16 on the Crew dragon. It will four times the thrust and six times the propellant. If there was ever a mission to move something as heavy as the space station to a higher orbit from low earth orbit then even more propellant would be needed. Three times the engines for four times the thrust indicates a 33% improvement in thrust for each engine.
SpaceX and NASA could choose to make larger crew Dragons. The Crew Dragon can hold up to seven people but it currently has only moved up to four astronauts. An orbital or Cis-lunar tug with more engines could be built to transport many dozens of people.
The architecture and systems used in Crew Dragon have been designed and tested for higher levels of safety.
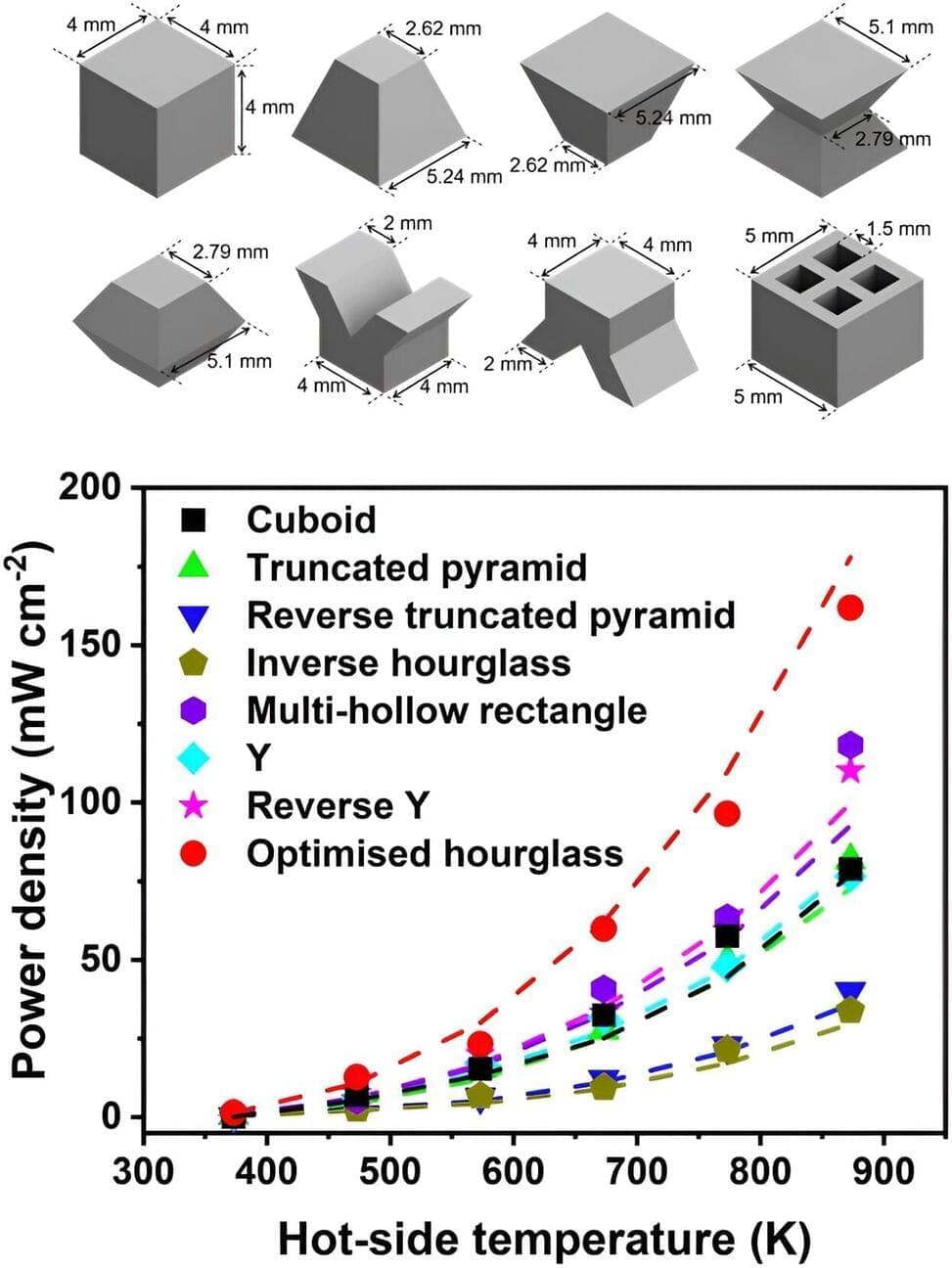
In recent years, engineers and scientists worldwide have been working on new technologies for generating electricity from renewable energy sources, including photovoltaics (PVs), wind turbines and hydro-power generators. An alternative solution for mitigating the impact of climate change could be to convert the excess or waste heat generated by industries, households and hot natural environments into electricity.
This approach, known as thermoelectric power generation, relies on the use of materials with valuable thermoelectric properties. Specifically, when these materials are exposed to particularly high temperatures on one side and colder ones on the other, electrons within them start to flow from the hot side to the cooler one, which generates electrical potential
While recent works have identified some promising thermoelectric materials, the module performance is unsatisfactory due to the challenges associated with designing and fabricating optimum module structures. This significantly limits their potential real-world integration in thermoelectric modules.