Researchers have shown for the first time that flipping an epigenetic “switch” in specific memory-holding neurons can directly alter memory strength.


“The ability to resolve the Gulf Stream and its dynamics properly, has been an open challenge for many years in oceanography,” said Dr. Ashesh Chattopadhyay.
How can AI be used to predict ocean forecasting? This is what a recent study published in the Journal of Geophysical Research Machine Learning and Computation hopes to address as a team of researchers investigated how AI can be used to predict short-and long-term trends in ocean dynamics. This study has the potential to help scientists and the public better understand new methods estimating long-term ocean forecasting, specifically with climate change increasing ocean temperatures.
For the study, the researchers presented a new AI-based modeling tool for predicting ocean dynamics for the Gulf of Mexico, which is a major trade route between the United States and Mexico. The goal of the tool is to build upon longstanding physics-based models that have traditionally been used for predicting ocean dynamics, including temperature and changes in temperature.
In the end, the researchers found that this new model demonstrates improved performance in predicting ocean dynamics, specifically for short-term intervals of 30 days, along with long-term intervals of 10 years. The team aspires to use this new tool for modeling ocean dynamics worldwide.

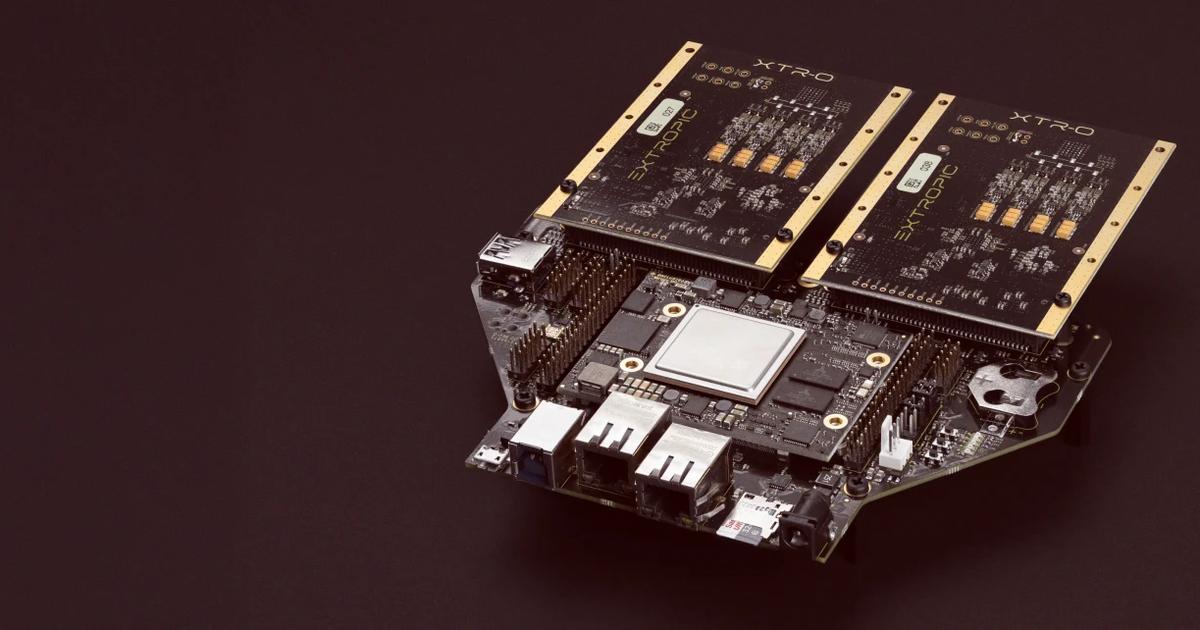
XTR-0 is the first way Extropic chips will be integrated with conventional computers. We intend to build more advanced systems around future TSUs that allow them to more easily integrate with conventional AI accelerators like GPUs. This could take the form of something simple like a PCIe card, or could in principle be as complicated as building a single chip that contains both a GPU and a TSU.
X0 houses a family of circuits that generate samples from primitive probability distributions. Our future chips will combine millions of these probabilistic circuits to run EBMs efficiently.
The probabilistic circuits on X0 output random continuous-time voltage signals. Repeatedly observing the signals (waiting sufficiently long between observations) allows a user to generate approximately independent samples from the distribution embodied by the circuit. These circuits are used to generate the random output voltage, making them much more energy efficient than their counterparts on deterministic digital computers.

Year 2015 face_with_colon_three
Researchers from MIT have created a 3D printer that is able to create objects out of glass.

Using state-of-the-art equipment, researchers in the Thermophysical Properties of Materials group from the University of the Basque Country (EHU) have analyzed the capacity of ultra-black copper cobaltate nanoneedles to effectively absorb solar energy. They showed that the new nanoneedles have excellent thermal and optical properties and are particularly suited to absorbing energy. This will pave the way toward concentrated solar power in the field of renewable energies.
The tests were carried out in a specialized lab that has the capacity to undertake high temperature research. The results were published in the journal Solar Energy Materials and Solar Cells.
Renewable energy of the future is concentrated solar power because it can be easily used to store thermal energy. Despite the fact that, historically, it is more expensive and complex than photovoltaic power, in recent years huge advances have taken place in this technology, and concentrated solar power plants are spreading across more and more countries as a resource for a sustainable future.
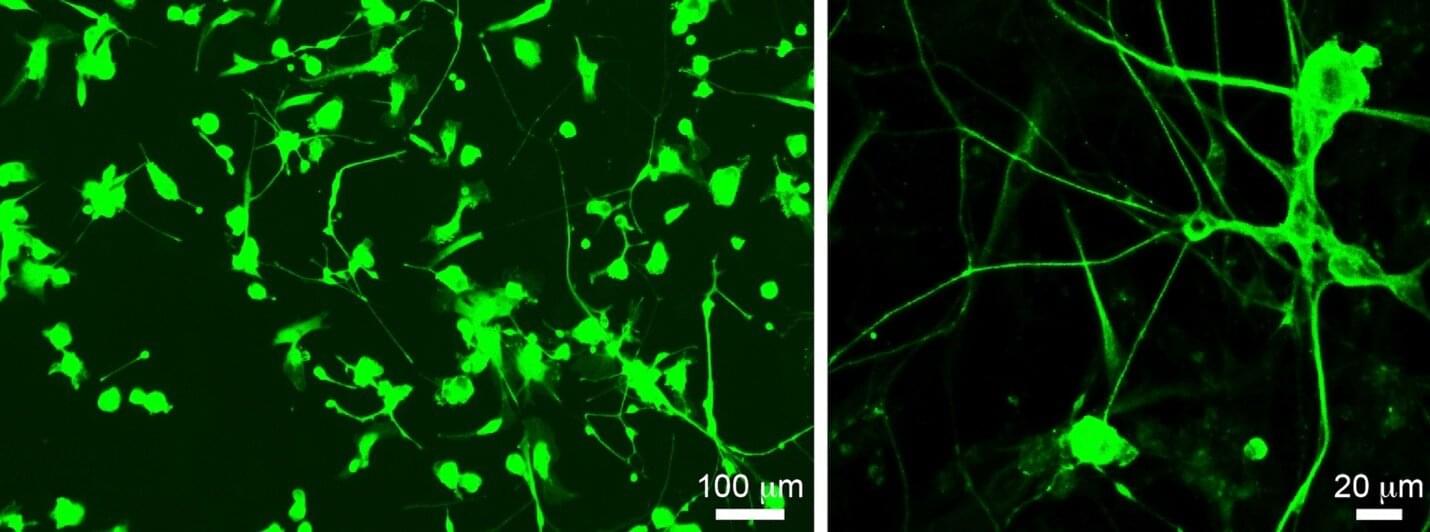
Spinal cord injury (SCI) remains a major unmet medical challenge, often resulting in permanent paralysis and disability with no effective treatments. Now, researchers at University of California San Diego School of Medicine have harnessed bioinformatics to fast-track the discovery of a promising new drug for SCI. The results will also make it easier for researchers around the world to translate their discoveries into treatments. The findings are published in the journal Nature.
One of the reasons SCI results in permanent disability is that the neurons that form our brain and spinal cord cannot effectively regenerate. Encouraging neurons to regenerate with drugs offers a promising possibility for treating these severe injuries.
The researchers found that under specific experimental conditions, some mouse neurons activate a specific pattern of genes related to neuronal growth and regeneration. To translate this fundamental discovery into a treatment, the researchers used data-driven bioinformatics approaches to compare their pattern to a vast database of compounds, looking for drugs that could activate these same genes and trigger neurons to regenerate.