Evidence that quantum information can get scrambled unconventionally in a chain of atoms could improve our understanding of quantum many-body dynamics.



Light can behave in very unexpected ways when you squeeze it into small spaces. In a paper in the journal Science, Mark Brongersma, a professor of materials science and engineering at Stanford University, and doctoral candidate Skyler Selvin describe the novel way they have used sound to manipulate light that has been confined to gaps only a few nanometers across—allowing the researchers exquisite control over the color and intensity of light mechanically.
The findings could have broad implications in fields ranging from computer and virtual reality displays to 3D holographic imagery, optical communications, and even new ultrafast, light-based neural networks.
The new device is not the first to manipulate light with sound, but it is smaller and potentially more practical and powerful than conventional methods. From an engineering standpoint, acoustic waves are attractive because they can vibrate very fast, billions of times per second.

The first black hole images stunned the world in 2019, with headlines announcing evidence of a glowing doughnut-shaped object from the center of galaxy Messier 87 (M87 —55 million light years from Earth. Supercomputer simulations are now helping scientists sharpen their understanding about the environment beyond a black hole’s ‘shadow,’ material just outside its event horizon.
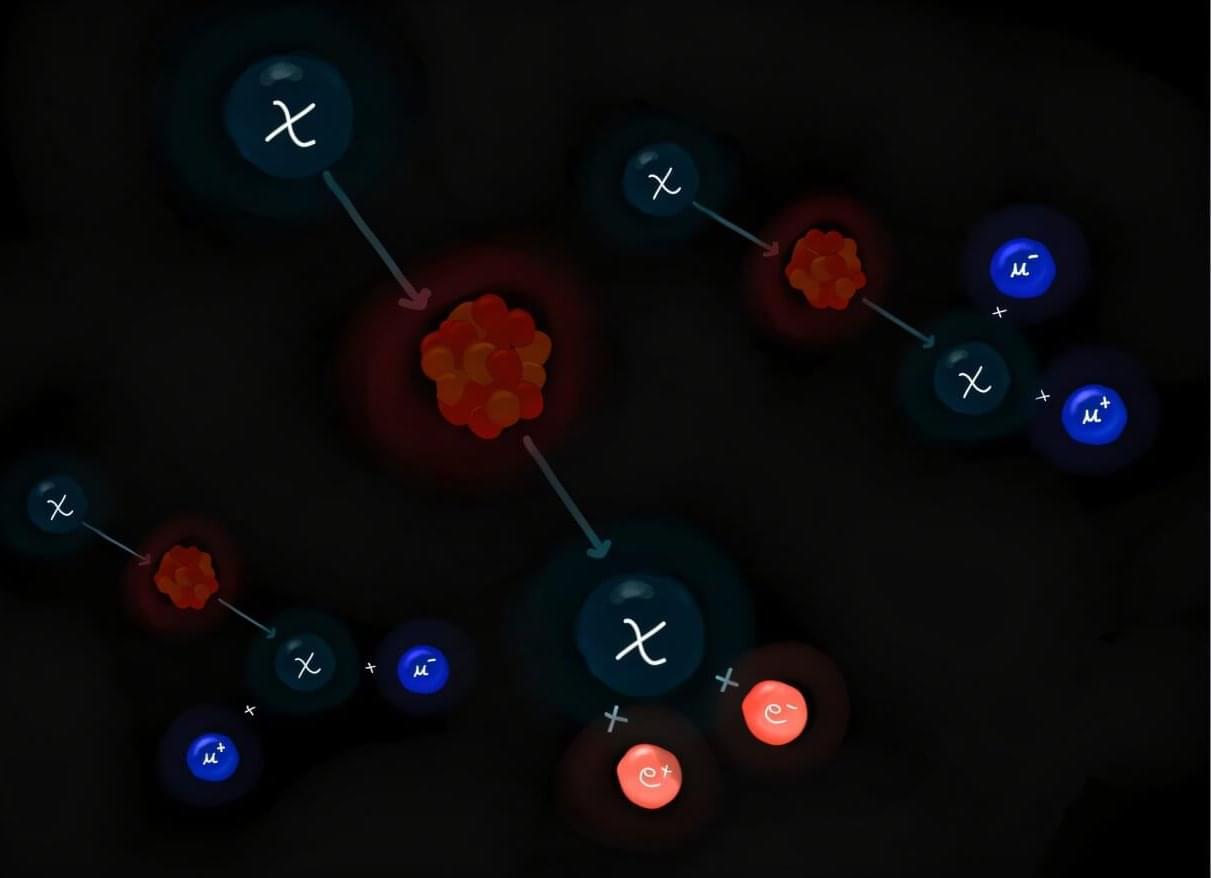
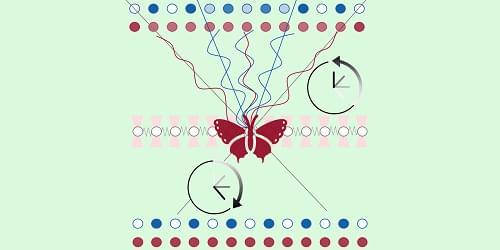
Dark matter (DM) is a type of matter estimated to account for 80% of the universe’s total mass, but it cannot be directly detected using conventional experimental techniques. As DM does not emit, reflect or absorb light, most previous dark matter searches were aimed at observing either its weak interactions with ordinary matter using highly sensitive detectors or other signatures linked to its presence or decay.
Researchers at Texas A&M University recently introduced a new approach that could enable the direct detection of this elusive type of matter, leveraging a process known as the DM internal pair production. Their proposed strategy, outlined in a paper published in Physical Review Letters, could open new possibilities for future DM searches focusing on a wide range of candidate particles.
“The particle nature of DM can be revealed when a DM particle scatters off a nucleus and produces a visible recoil signal,” the authors told Phys.org. “However, for light DM, transferring sufficient energy to a heavy nucleus is kinematically challenging, even if the DM is energetic. To overcome this limitation, we developed a framework where additional particles are produced in the final state, allowing the DM’s energy to be shared among them, while the nucleus remains largely at rest.”

Salt creeping, a phenomenon that occurs in both natural and industrial processes, describes the collection and migration of salt crystals from evaporating solutions onto surfaces. Once they start collecting, the crystals climb, spreading away from the solution. This creeping behavior, according to researchers, can cause damage or be harnessed for good, depending on the context.
New research published June 30 in the journal Langmuir is the first to show salt creeping at a single-crystal scale and beneath a liquid’s meniscus.
“The work not only explains how salt creeping begins, but why it begins and when it does,” says Joseph Phelim Mooney, a postdoc in the MIT Device Research Laboratory and one of the authors of the new study. “We hope this level of insight helps others, whether they’re tackling water scarcity, preserving ancient murals, or designing longer-lasting infrastructure.”

A research team at the Politecnico di Milano has developed an innovative single-atom catalyst capable of selectively adapting its chemical activity. This is a crucial step forward in sustainable chemistry and the design of more efficient and programmable industrial processes.
The study was published in the Journal of the American Chemical Society.
This achievement is novel in the field of single-atom catalysts. For the first time, scientists have demonstrated the possibility of designing a material that can selectively change its catalytic function depending on the chemical environment. It involves a sort of “molecular switch” that allows complex reactions to be performed more cleanly and efficiently, using less energy than conventional processes.
To further reduce the size of electronic devices, while also improving their performance and energy efficiency, electronics engineers have been trying to identify alternative materials that outperform silicon and other conventional semiconductors. Two-dimensional (2D) semiconductors, materials that are just a few atoms thick and have a tunable electrical conductivity, are among the most promising candidates for the fabrication of smaller and better performing devices.
Past studies showed that these materials could be used to fabricate miniaturized transistors, electronic components that amplify or switch electrical signals, particularly field-effect transistors (FETs). These are transistors that control the flow of electrical current using an electric field.
To reliably operate, however, FETs also need to integrate an insulating layer that separates the so-called gate electrode (i.e., the terminal regulating the flow of current) from the channel (i.e., the pathway through which electrical current flows). To enable greater control over the gate, this insulating layer, known as a gate dielectric, should have a high dielectric constant (κ), or in other words, it should effectively store electrical energy.