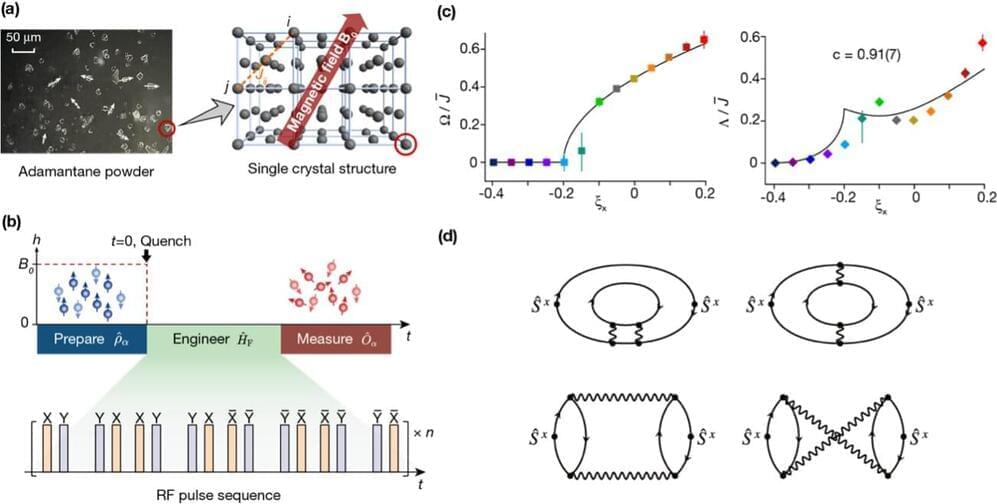
A new study has uncovered the universal dynamics far from equilibrium in randomly interacting spin models, thereby complementing the well-established universality in low-energy equilibrium physics. The study, recently published in Nature Physics, was the result of a collaborative effort involving the research group led by Prof. Du Jiangfeng and Prof. Peng Xinhua at the University of Science and Technology of China (USTC), along with the theoretical groups of Prof. Zhai Hui from Tsinghua University and Dr. Zhang Pengfei from Fudan University.