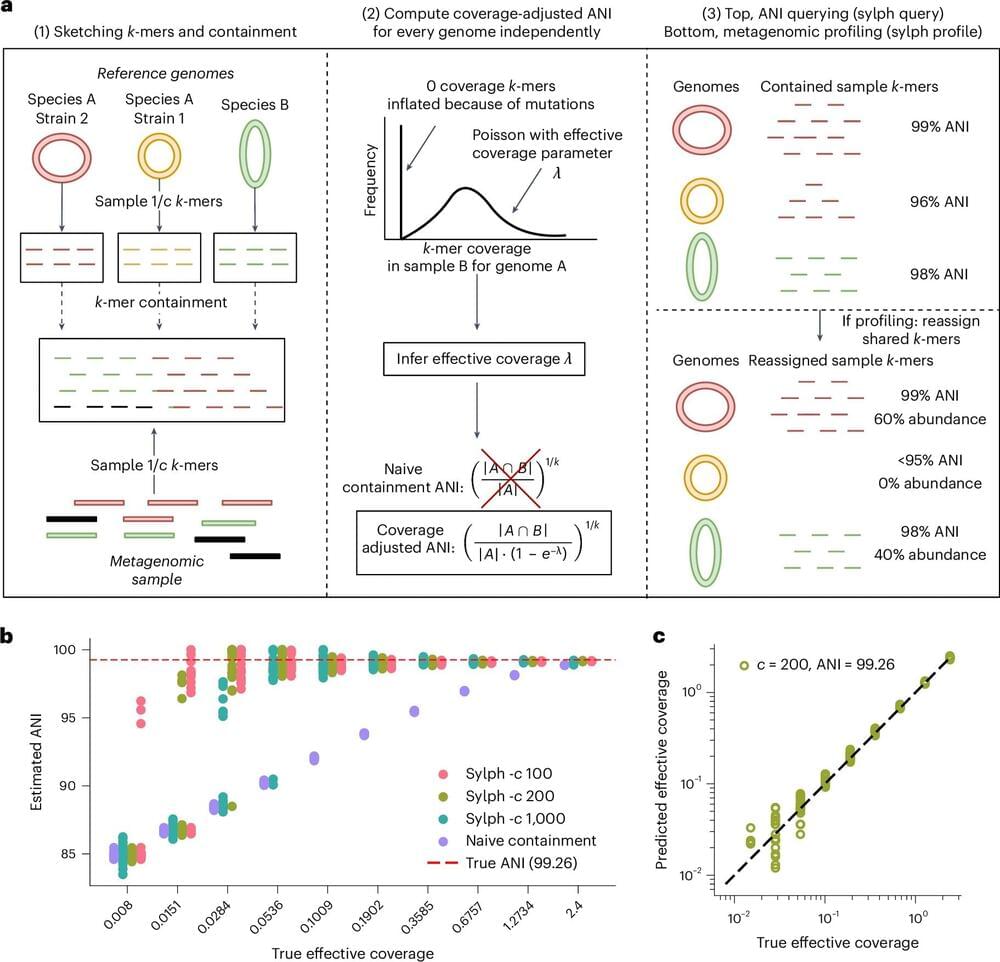
Researchers at Carnegie Mellon University and the University of Toronto have developed a new k-mer sketching metagenomic profiler, called sylph, that allows scientists to analyze genomic data more quickly and precisely than other profilers.

“The last two decades saw software transform nearly every industry, a trend famously captured by venture capitalist Marc Andreessen’s phrase ” software is eating the world.
Software became the backbone of industries like retail, transportation, finance, and entertainment, leading to a world where digital tools and applications are integral to daily life.
Looking forward, many experts believe that artificial intelligence (AI) will play a similarly transformative role over the next 20 years.

An asteroid struck Mars 11 million years ago and sent pieces of the red planet hurtling through space. One of these chunks of Mars eventually crashed into the Earth somewhere near Purdue University and is one of the few meteorites that can be traced directly to Mars. This meteorite was rediscovered in a drawer at Purdue University in 1931 and named the Lafayette Meteorite.

Johns Hopkins University-led researchers, working with the Biomarkers for Older Controls at Risk for Dementia (BIOCARD) cohort, have found that certain factors are linked to faster brain shrinkage and quicker progression from normal thinking abilities to mild cognitive impairment (MCI). People with type 2 diabetes and low levels of specific proteins in their cerebrospinal fluid showed more rapid brain changes and developed MCI sooner than others.
Long-term studies tracking brain changes over many years are rare but valuable. Previous research mostly provided snapshots in time, which can’t show how individual brains change over the years. By following participants for up to 27 years (20-year median), this study offers new insights into how health conditions might speed up brain aging.
In a study, “Acceleration of Brain Atrophy and Progression From Normal Cognition to Mild Cognitive Impairment,” published in JAMA Network Open, researchers used the BIOCARD cohort to examine risk factors associated with the acceleration of brain atrophy and progression from normal cognition to MCI. An Invited Commentary is also available.
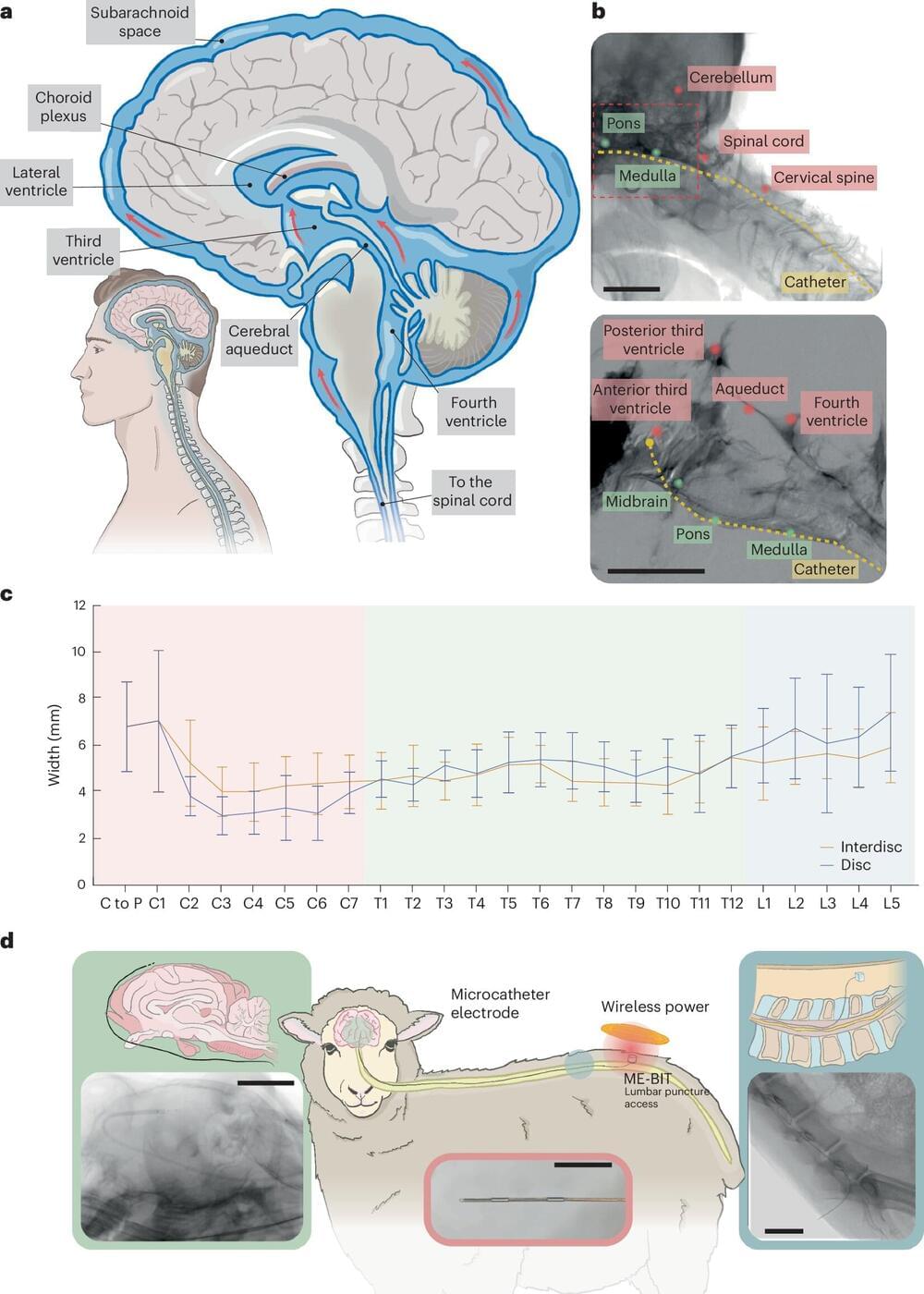
A team of researchers led by Rice University’s Jacob Robinson and the University of Texas Medical Branch’s Peter Kan has developed a technique for diagnosing, managing and treating neurological disorders with minimal surgical risks. The team’s findings were published in Nature Biomedical Engineering.
While traditional approaches for interfacing with the nervous system often require creating a hole in the skull to interface with the brain, the researchers have developed an innovative method known as endocisternal interfaces (ECI), allowing for electrical recording and stimulation of neural structures, including the brain and spinal cord, through cerebral spinal fluid (CSF).
“Using ECI, we can access multiple brain and spinal cord structures simultaneously without ever opening up the skull, reducing the risk of complications associated with traditional surgical techniques,” said Robinson, professor of electrical and computer engineering and bioengineering.
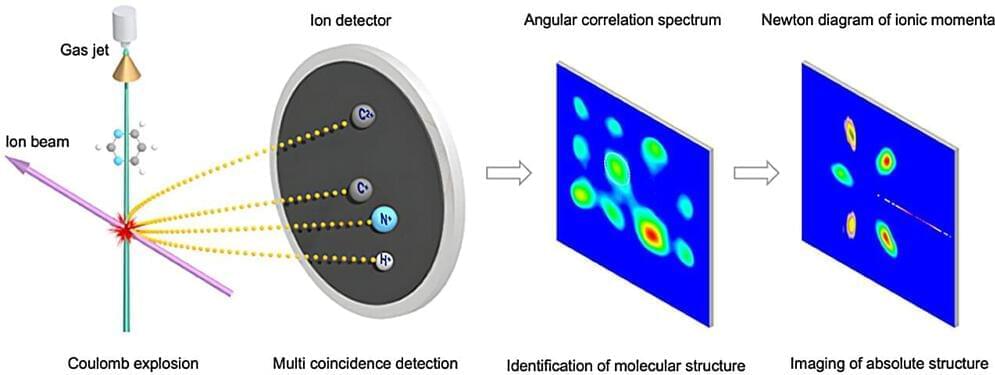
A new study published in Physical Review Letters and led by researchers from the Institute of Modern Physics (IMP) of the Chinese Academy of Sciences (CAS) has demonstrated that a Coulomb explosion induced by highly charged ions is a unique tool for precisely imaging the structures of complex molecules.