Over 300 live samples of deadly viruses—including hantavirus, lyssavirus and Hendra virus—went unaccounted for after a freezer broke.



The Philippine authorities ordered nearly 90,000 people to evacuate after a volcano on the central island of Negros erupted, spewing a two-mile-high plume of deadly ash, lava and large rocks.
The volcano, Mount Kanlaon, erupted on Monday afternoon, and the authorities warned that it could happen again in the coming days.
“This is very destructive and it can burn everything in its path, including vegetation, buildings and humans,” said Teresito Bacolcol, the head of seismology at the Philippine Institute of Volcanology and Seismology. “This can kill.”

A team of Indian astronomers has made a fascinating discovery that could change how we think about how planets are born. The team, led by Liton Majumdar from the National Institute of Science Education and Research (NISER) in Odisha, studied a unique triple-star system called GG Tau A, located 489 light-years away from Earth, as mentioned in the latest report by India Today.
Synchron has developed a Brain-Computer Interface that uses pre-existing technologies such as the stent and catheter to allow insertion into the brain without the need for open brain surgery.
Read the CNET article for more info:
You Might Not Need Open Brain Surgery to Get Mind Control https://cnet.co/3sZ7k67
0:00 Intro.
0:25 History of Brain Chip Implants.
0:44 About Synchron.
0:54 How Synchron implants the interface.
1:55 How brain patterns transmit signals.
2:50 Risks and Concerns.
3:50 Patients and Clinical Testing.
4:25 Brain Health Monitoring.
5:04 Synchron Switch Price.
Never miss a deal again! See CNET’s browser extension 👉 https://bit.ly/3lO7sOU
Check out CNET’s Amazon Storefront: https://www.amazon.com/shop/cnet?tag=lifeboatfound-20.
Follow us on TikTok: / cnetdotcom.
Follow us on Instagram: / cnet.
Follow us on Twitter: / cnet.
Like us on Facebook: / cnet.
#WhatTheFuture #Synchron #BCI

Bacancy is proud to announce the launch of a new AI tool, MedPreGPT, to help doctors with medicine prescriptions. This system enables doctors who all are using it for internal purposes to give accurate prescription recommendations privately without wasting any time. Compared to AI models like ChatGPT, this tool is specially trained with vast medical data and makes relevant suggestions. This innovative tool is made to enhance patient care, reduce human errors, and streamline the prescription process.
Doctors are only humans and they indeed work under intense work pressure and workload. Mistakes can happen in such an environment. Today’s healthcare providers use ChatGPT and Google’s Gemini for medicine recommendations, which is not completely wrong, but those tools might give false information. Bacancy has recognized the problem and found MedPreGPT to give accurate medical prescriptions.
The following are features of MedPreGPT Provides AI-based prescription recommendations according to symptoms and history. It is integrated with electronic health records (EHRs) for workflow ease. It provides multilingual support for healthcare professionals across the world Provides healthcare providers with updates in real time, regarding the latest clinical guidelines and drug interactions to ensure true care.
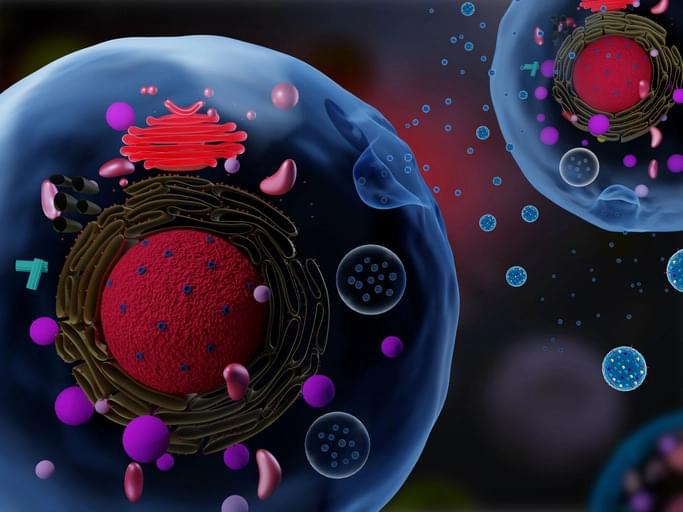

In a recent study published in Nature Cancer, a team of researchers investigated the unique structure of deoxyribonucleic acid (DNA) in extracellular vesicles (EVs) and its role in cancer progression.
The study examined how EV-DNA, through its association with histones, influences immune cell responses and impacts the pre-metastatic niche. It also explored how it could serve as a predictive biomarker for metastasis, especially in colorectal cancer.
The study highlights how EV-DNA packaging influences immune responses and its promise as a biomarker for assessing colorectal cancer metastasis risk.
Increasing the levels of chemicals naturally produced in the body called endocannabinoids may thwart the highly addictive nature of opioids such as morphine and oxycodone while maintaining the drugs’ ability to relieve pain, according to Weill Cornell Medicine investigators working with researchers from The Center for Youth Mental Health at NewYork-Presbyterian. Endocannabinoids bind to cannabinoid receptors throughout the body that regulate activities, such as learning and memory, emotions, sleep, immune response and appetite.
Opioids prescribed to control pain can become addictive because they not only dull pain, but also produce a sense of euphoria. The preclinical study, published Nov. 29 in Science Advances, may lead to a new type of therapeutic that could be taken with an opioid regimen to only reduce the rewarding aspect of opioids.
In 2023, opioid abuse or overuse was responsible for more than 80,000 deaths, fueling a national crisis, according to the U.S. Centers for Disease Control and Prevention. Illegal recreational drugs were ultimately responsible for many deaths, but not all of them. “When someone has surgery and is taking opioids for pain management, there’s always a risk of developing a dependence on these drugs,” said senior author Dr. Francis Lee, chair of the Department of Psychiatry at Weill Cornell Medicine and psychiatrist-in-chief at New York-Presbyterian/Weill Cornell Medical Center.
Drug-induced toxicity is one of the leading reasons new drugs fail clinical trials. Machine learning models that predict drug toxicity from molecular structure could help researchers prioritize less toxic drug candidates. However, current toxicity datasets are typically small and limited to a single organ system (e.g., cardio, renal, or liver). Creating these datasets often involved time-intensive expert curation by parsing drug label documents that can exceed 100 pages per drug. Here, we introduce UniTox[1][1], a unified dataset of 2,418 FDA-approved drugs with drug-induced toxicity summaries and ratings created by using GPT-4o to process FDA drug labels. UniTox spans eight types of toxicity: cardiotoxicity, liver toxicity, renal toxicity, pulmonary toxicity, hematological toxicity, dermatological toxicity, ototoxicity, and infertility. This is, to the best of our knowledge, the largest such systematic human in vivo database by number of drugs and toxicities, and the first covering nearly all FDA-approved medications for several of these toxicities. We recruited clinicians to validate a random sample of our GPT-4o annotated toxicities, and UniTox’s toxicity ratings concord with clinician labelers 87–96% of the time. Finally, we benchmark a graph neural network trained on UniTox to demonstrate the utility of this dataset for building molecular toxicity prediction models.
### Competing Interest Statement.
The authors have declared no competing interest.