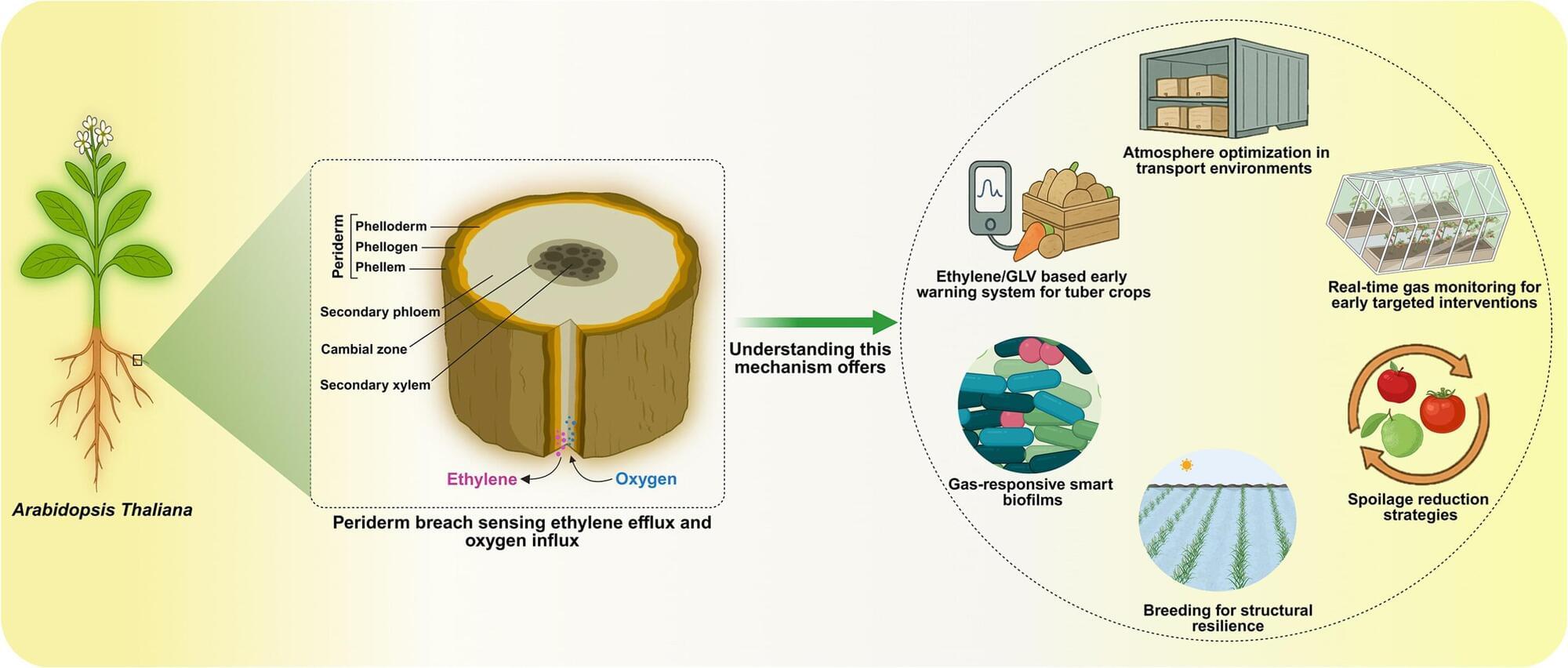
In the predawn darkness, a team of scientists climbs the slope of Mexico’s Popocatépetl volcano, one of the world’s most active and whose eruption could affect millions of people. Its mission: figure out what is happening under the crater.
For five years, the group from Mexico’s National Autonomous University has climbed the volcano with kilos of equipment, risked data loss due to bad weather or a volcanic explosion and used artificial intelligence to analyze the seismic data. Now, the team has created the first three-dimensional image of the 17,883-foot (5,452-meter) volcano’s interior, which tells them where the magma accumulates and will help them better understand its activity, and, eventually, help authorities better react to eruptions.
Marco Calò, professor in the UNAM’s Geophysics Institute’s vulcanology department and the project leader, invited The Associated Press to accompany the team on its most recent expedition, the last before its research on the volcano will be published.