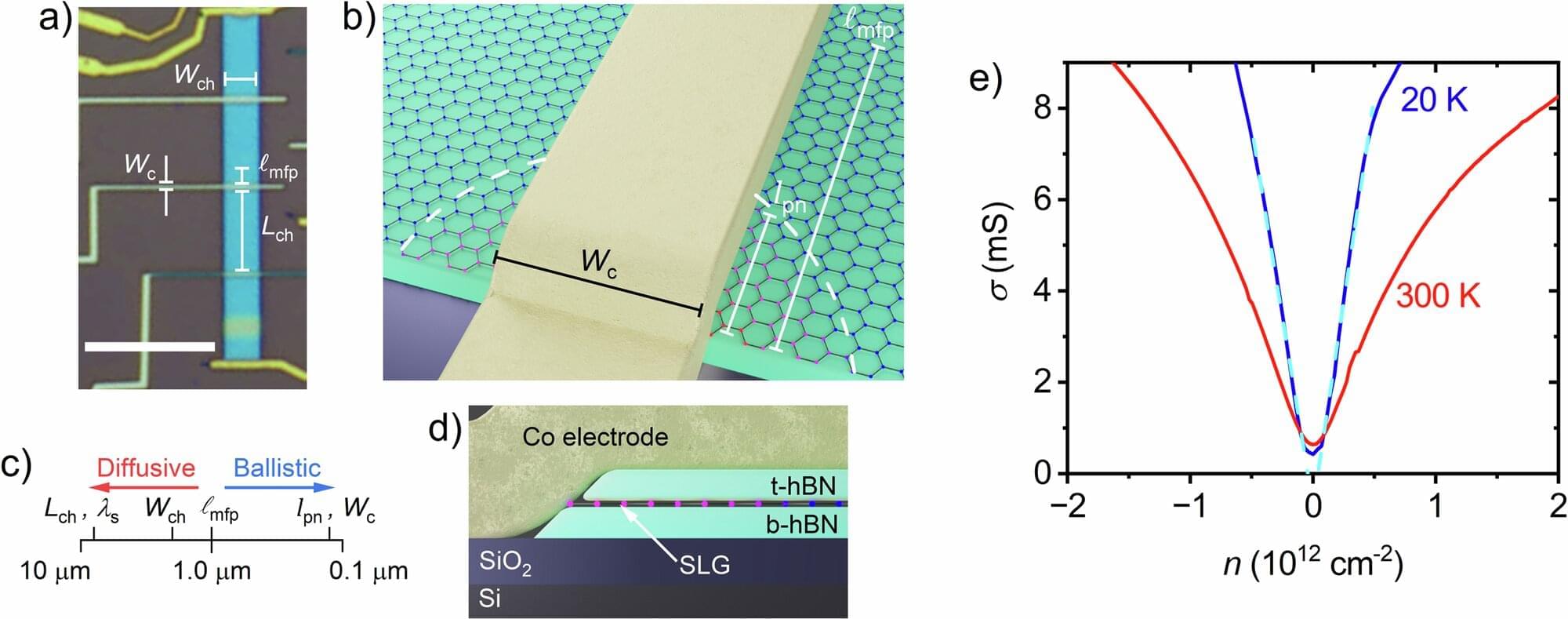
MIT physicists report the unexpected discovery of electrons forming crystalline structures in a material only billionths of a meter thick. The work adds to a gold mine of discoveries originating from the material, which the same team discovered only about three years ago.
In a paper published Jan. 22 in Nature, the team describes how electrons in devices made, in part, of the new material can become solid, or form crystals, by changing the voltage applied to the devices when they are kept at a temperature similar to that of outer space. Under the same conditions, they also showed the emergence of two new electronic states that add to work they reported last year showing that electrons can split into fractions of themselves.
The physicists were able to make the discoveries thanks to new custom-made filters for better insulation of the equipment involved in the work. These allowed them to cool their devices to a temperature an order of magnitude colder than they achieved for the earlier results.