Google faces $34.5 billion challenge as AI rival perplexity makes play for Chrome.
Perplexity AI’s $34.5 billion cash bid for Google’s Chrome aims to reshape the AI search and browser landscape.




Cobras kill thousands of people a year worldwide and perhaps a hundred thousand more are seriously maimed by necrosis – the death of body tissue and cells – caused by the venom, which can lead to amputation.
Current antivenom treatment is expensive and does not effectively treat the necrosis of the flesh where the bite occurs.
“Our discovery could drastically reduce the terrible injuries from necrosis caused by cobra bites – and it might also slow the venom, which could improve survival rates,” said Professor Greg Neely, a corresponding author of the study from the Charles Perkins Centre and Faculty of Science at the University of Sydney.

Georgetown Lombardi, Washington’s only National Cancer Institute-designated Comprehensive Cancer Center, serves as the research engine for MedStar Health, Georgetown University’s clinical partner. Georgetown Lombardi is also an NCI-recognized research consortium with John Theurer Cancer Center of Hackensack Meridian Health in Bergen County, New Jersey.
They have a blog with alot of useful cancer information you can share. There is info about them circulating about cancer vaccines and clinical trials. Check em out:
(You can repost their posts or contact them to recieve information from them directly. Lombardi Comprehensive Cancer Center 3,800 Reservoir Rd. NW Washington D.C. 20,057 Phone: 202−444−2223)

A study published in Nature Biotechnology reveals a powerful new use for artificial intelligence: designing small, drug-like molecules that can stick to and break down harmful proteins in the body — even when scientists don’t know what those proteins look like. The breakthrough could lead to new treatments for diseases that have long resisted traditional drug development, including certain cancers, brain disorders, and viral infections.
The study was published on August 13, 2025 by a multi-institutional team of researchers from McMaster University, Duke University, and Cornell University. The AI tool, called PepMLM, is based on an algorithm originally built to understand human language and used in chatbots, but was trained to understand the “language” of proteins.
In 2024, the Nobel Prize in Chemistry was awarded to researchers at Google DeepMind for developing AlphaFold, an AI system that predicts the 3D structure of proteins – a major advance in drug discovery. But many disease-related proteins, including those involved in cancer and neurodegeneration, don’t have stable structures. That’s where PepMLM takes a different approach – instead of relying on structure, the tool uses only the protein’s sequence to design peptide drugs. This makes it possible to target a much broader range of disease proteins, including those that were previously considered “undruggable.”
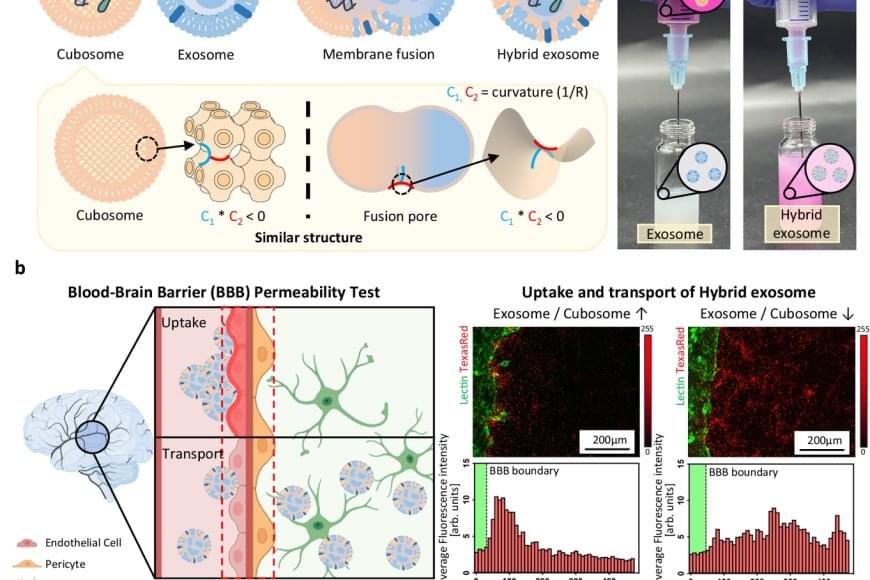
Exosomes, naturally derived vesicles responsible for intercellular communication, are emerging as next-generation drug delivery systems capable of transporting therapeutics to specific cells. However, their tightly packed, cholesterol-rich membranes make it extremely difficult to encapsulate large molecules such as mRNA or proteins.
Conventional approaches have relied on techniques like electroporation or chemical treatment, which often damage both the drugs and exosomes, reduce delivery efficiency, and require complex purification steps—all of which pose significant barriers to commercialization.
The team utilized a lipid-based nanoparticle known as a “cubosome,” which mimics the fusion structure of cell membranes and naturally fuses with exosomes. By mixing cubosomes carrying mRNA with exosomes at room temperature for just 10 minutes, the researchers achieved efficient fusion and confirmed that the mRNA was successfully loaded into the exosomes. Analysis showed that over 98% of the mRNA was encapsulated, while the structural integrity and biological function of the exosomes were preserved.
Furthermore, the engineered exosomes demonstrated the ability to cross the blood-brain barrier, one of the most difficult hurdles in drug delivery. Notably, the team observed a “homing” effect, where exosomes return to the type of cell they originated from, enabling targeted drug delivery to diseased tissues.
