Exosomes, naturally derived vesicles responsible for intercellular communication, are emerging as next-generation drug delivery systems capable of transporting therapeutics to specific cells. However, their tightly packed, cholesterol-rich membranes make it extremely difficult to encapsulate large molecules such as mRNA or proteins.
Conventional approaches have relied on techniques like electroporation or chemical treatment, which often damage both the drugs and exosomes, reduce delivery efficiency, and require complex purification steps—all of which pose significant barriers to commercialization.
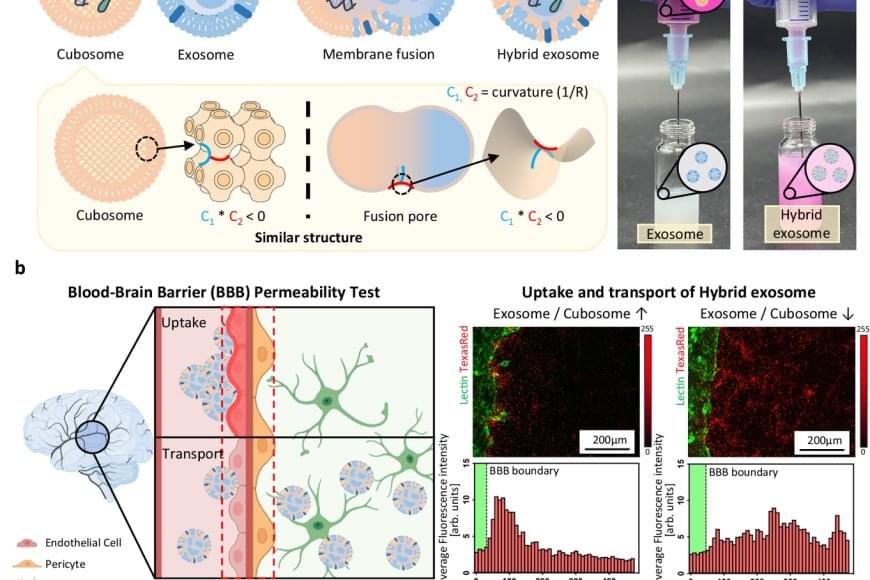
The team utilized a lipid-based nanoparticle known as a “cubosome,” which mimics the fusion structure of cell membranes and naturally fuses with exosomes. By mixing cubosomes carrying mRNA with exosomes at room temperature for just 10 minutes, the researchers achieved efficient fusion and confirmed that the mRNA was successfully loaded into the exosomes. Analysis showed that over 98% of the mRNA was encapsulated, while the structural integrity and biological function of the exosomes were preserved.
Furthermore, the engineered exosomes demonstrated the ability to cross the blood-brain barrier, one of the most difficult hurdles in drug delivery. Notably, the team observed a “homing” effect, where exosomes return to the type of cell they originated from, enabling targeted drug delivery to diseased tissues.