Tissue-resident memory T cells that express TCF1 or ID3 exhibit stem-like properties and regulate memory heterogeneity and recall.



“This is strong evidence that as stars evolve off their main sequence they can quickly cause planets to spiral into them and be destroyed,” said Dr. Edward Bryant.
What happens to planets as their stars age and come closer to death? This is what a recent study published in the Monthly Notices of the Royal Astronomical Society hopes to address as a team of researchers investigated the interaction between stars near the end of their lifetimes and their exoplanets with short-period orbits. This study has the potential to help scientists better understand the evolution of stars and what this could mean for our Sun near the end of its lifetime.
For the study, the researchers analyzed data obtained from NASA’s Transiting Exoplanet Survey Satellite (TESS) mission for short-period exoplanets orbiting post-main-sequence stars, which are stars approximately the size of our Sun which have exhausted their hydrogen and have ballooned into red giants. Additionally, these short-period exoplanets have orbits that last mere days.
The goal of the study was to ascertain the influence of these red giants on their planetary populations, with the researchers settling on 130 exoplanets after careful data analysis. In the end, the researchers found that only 0.28 percent of older post-main sequence stars had giant exoplanets, with 0.35 percent of younger post-main-sequence stars having giant exoplanets. Finally, the researchers found only 0.11 percent of the oldest post-main-sequence stars had exoplanets.
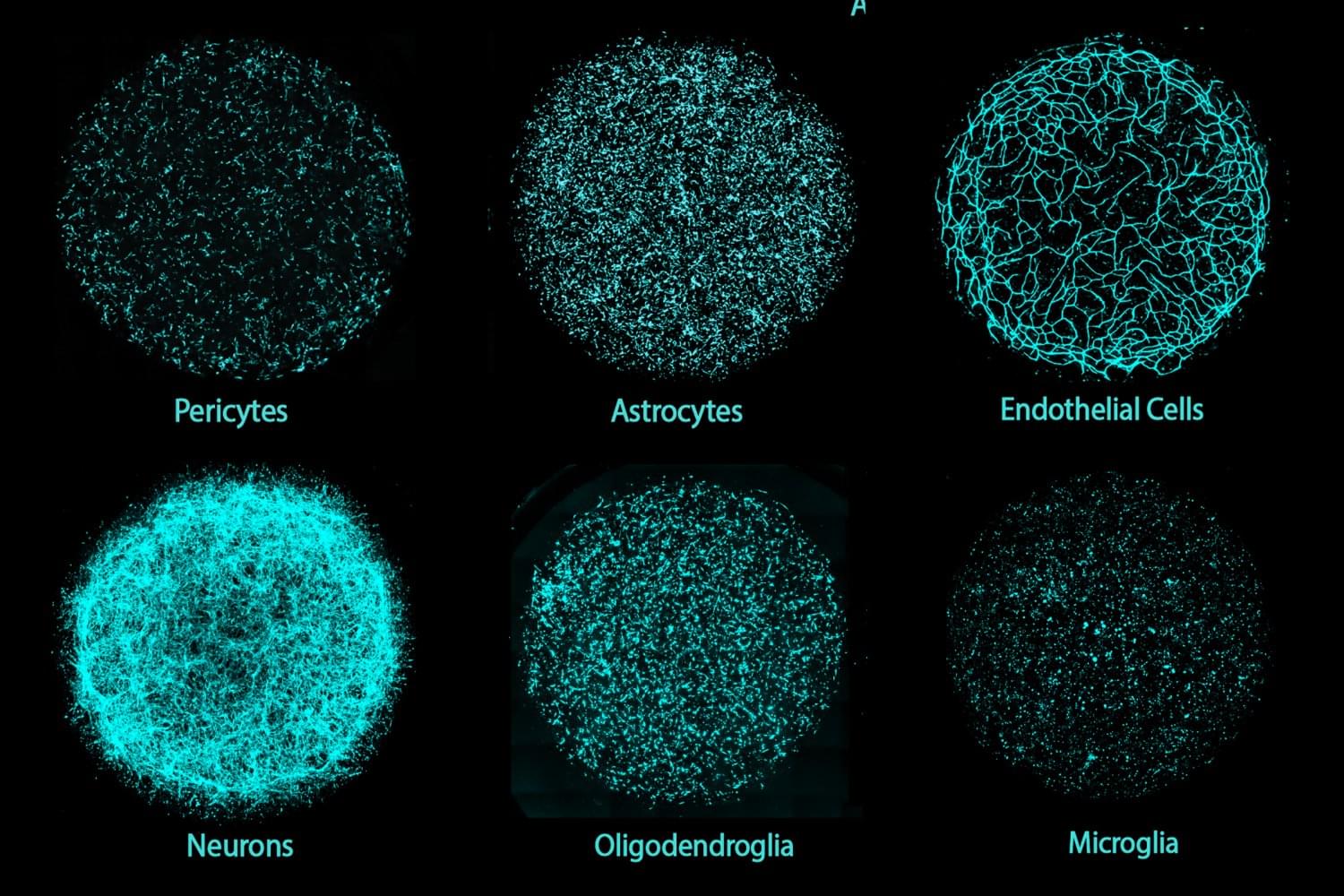
A new 3D human brain tissue platform developed by MIT researchers is the first to integrate all major brain cell types, including neurons, glial cells, and the vasculature, into a single culture.
Grown from individual donors’ induced pluripotent stem cells, these models — dubbed Multicellular Integrated Brains (miBrains) — replicate key features and functions of human brain tissue, are readily customizable through gene editing, and can be produced in quantities that support large-scale research.
Although each unit is smaller than a dime, miBrains may be worth a great deal to researchers and drug developers who need more complex living lab models to better understand brain biology and treat diseases.


Neuroblastoma RAS viral oncogene homolog (NRAS)-mutant melanoma is an aggressive form of skin cancer that develops because of a RAS genetic mutation within the cells. It is a common mutation in melanoma and accounts for 15–20% of melanoma diagnoses. An individual has greater risk of melanoma when exposed to the sun for extended periods of time. Additionally, individuals may have family history of melanoma that would increase their risk. It is important to regularly visit the dermatologist to confirm pigmented or non-pigmented moles are not cancerous. To evaluate each mole doctor’s access the change in shape, color, size, and unusual growth as time progresses.
Melanoma, specifically the NRAS subtype, can grow rapidly and spread to other areas of the body. Symptoms may also include change in nail appearance, eye issues, and mouth sores. While dependent on the stage of cancer, treatment usually includes drugs that target the NRAS pathway. Immunotherapeutic approaches include a checkpoint inhibitor treatment that activates immune cell response. Other forms of treatment include surgery and combination therapy. Scientists are working to learn more about NRAS melanoma and how to develop better treatments. Recent work has shown particular promise of treating NRAS melanoma in the lab and clinic.
A recent article in Cancer Immunology Research, by Dr. Keiran Smalley and others, demonstrated that blocking the RAS pathway in NRAS-mutated melanoma cells limit tumor growth and expansion. Smalley is a Professor and Scientific Director in the Donald A. Adam Comprehensive Melanoma Research Center of Excellence at Moffitt Cancer Center. His work focuses on understanding melanoma and the immune response after therapeutic treatment. In addition, Smalley is interested in using computational biology and other techniques to not only assess therapeutic benefit but develop novel treatments specific to mutated melanoma cells.
The innovation is claimed to be ideal for cold-climate electronics, wearable devices, and grid storage.
Researchers have demonstrated that aqueous zinc-ion batteries can offer long-term cycling stability and higher energy density with a new method.
Researchers from The Hong Kong Polytechnic University and Shenzhen University used a different type of cathode that delivers exceptional performance in aqueous zinc-ion batteries across a wide temperature range.
They developed a novel K⁺ and C3N4 co-intercalated NH4V4O10 (KNVO-C3N4) cathode to use in aqueous zinc-ion batteries.

Researchers from Nobel Laureate David Baker’s lab and the University of Washington’s Institute for Protein Design (IPD) have used artificial intelligence to design antibodies from scratch — notching another game-changing breakthrough for the scientists and their field of research.
“It was really a grand challenge — a pipe dream,” said Andrew Borst, head of electron microscopy R&D at IPD. Now that they’ve hit the milestone of engineering antibodies that successfully bind to their targets, the research “can go on and it can grow to heights that you can’t imagine right now.”
Borst and his colleagues are publishing their work in the peer-reviewed journal Nature. The development could supercharge the $200 billion antibody drug industry.
