The patient had been undergoing treatment since early November.


RAY-lee) is the scattering or deflection of light, or other electromagnetic radiation, by particles with a size much smaller than the wavelength of the radiation. For light frequencies well below the resonance frequency of the scattering medium (normal dispersion regime), the amount of scattering is inversely proportional to the fourth power of the wavelength (e.g., a blue color is scattered much more than a red color as light propagates through air). The phenomenon is named after the 19th-century British physicist Lord Rayleigh (John William Strutt). [ 1 ].

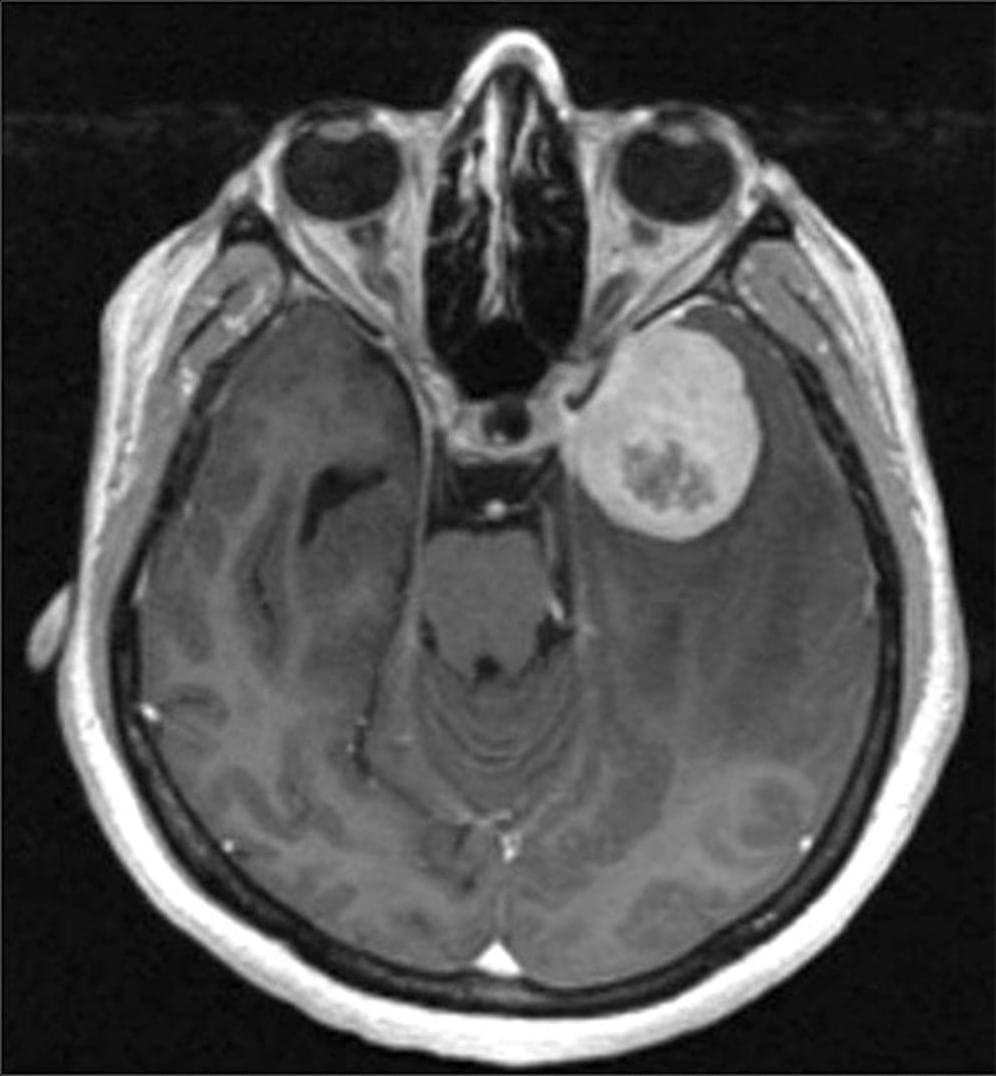
Clinicians typically classify meningiomas — the most common type of brain tumor — into three grades, ranging from slow-growing to aggressive.
But a new multi-institutional study suggests that appearances may be deceiving. If a tumor shows activity in a gene called telomerase reverse transcriptase (TERT), it tends to recur more quickly, even if it looks low-grade under the microscope.
Researchers discover that when meningiomas, a type of brain tumor, shows activity in the TERT gene, it tends to recur more quickly.

Nvidia CEO Jensen Huang says he uses AI chatbots like OpenAI’s ChatGPT or Google’s Gemini to write his first drafts for him.

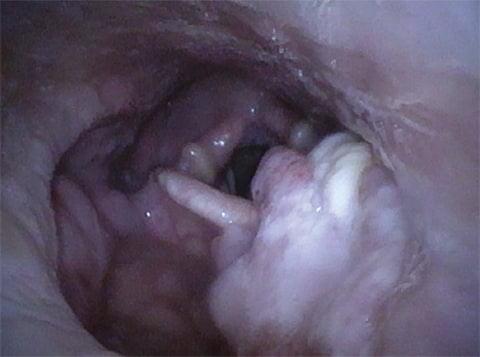
Case Report:
Lichen planus is an inflammatory disorder of immune dysregulation that affects the skin and mucosa. Oral lichen planus (OLP) is a chronic variant characterized by white mucosal lesions,1 most commonly with bilateral buccal mucosa involvement and frequently involving the tongue and gingiva as well.2 Although the underlying cause remains obscure, OLP is thought to have an autoimmune etiology and has been linked with genetic factors, hypertension, diabetes mellitus, hepatitis C virus, and thyroid dysfunction.3
OLP onset involves the activation of immune pathways leading to migration and activation of T cells and the destruction of keratinocytes.4 It is thought that oral mucosal keratinocytes are activated by the expression of unknown antigens, which recruit lymphocytes. This T-cell-mediated response is coupled with the simultaneous nonspecific response of matrix metalloproteases, chemokines and mast cells, together causing apoptosis of the basal keratinocytes by various mechanisms.
OLP can undergo malignant transformation to oral squamous cell carcinoma (OSCC) in a small subset of OLP patients (1%), more commonly in smokers, alcoholics, and hepatitis C patients.5 It is thus considered an OSCC precursor lesion. Topical steroids are the first-line treatment, but systemic steroids and topical calcineurin inhibitors can be used to manage recalcitrant cases.6
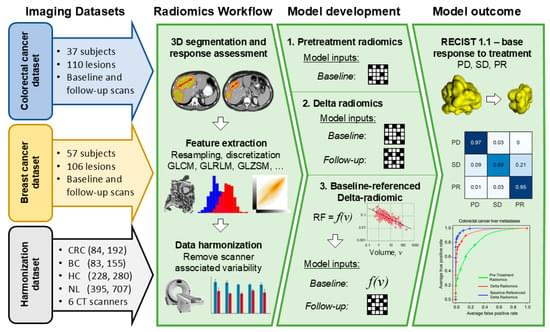
Background/Objectives: Radiomic features exhibit a correlation with tumor size on pretreatment images. However, on post-treatment images, this association is influenced by treatment efficacy and varies between responders and non-responders. This study introduces a novel model, called baseline-referenced Delta radiomics, which integrates the association between radiomic features and tumor size into Delta radiomics to predict chemotherapy response in liver metastases from breast cancer (BC) and colorectal cancer (CRC). Materials and Methods: A retrospective study analyzed contrast-enhanced computed tomography (CT) scans of 83 BC patients and 84 CRC patients. Among these, 57 BC patients with 106 liver lesions and 37 CRC patients with 109 lesions underwent post-treatment imaging after systemic chemotherapy. Radiomic features were extracted from up to three lesions per patient following manual segmentation. Tumor response was assessed by measuring the longest diameter and classified according to RECIST 1.1 criteria as progressive disease (PD), partial response (PR), or stable disease (SD). Classification models were developed to predict chemotherapy response using pretreatment data only, Delta radiomics, and baseline-referenced Delta radiomics. Model performance was evaluated using confusion matrix metrics. Results: Baseline-referenced Delta radiomics performed comparably or better than established radiomics models in predicting tumor response in chemotherapy-treated patients with liver metastases. The sensitivity, specificity, and balanced accuracy in predicting response ranged from 0.66 to 0.97, 0.81 to 0.97, and 80% to 90%, respectively. Conclusions: By integrating the relationship between radiomic features and tumor size into Delta radiomics, baseline-referenced Delta radiomics offers a promising approach for predicting chemotherapy response in liver metastases from breast and colorectal cancer.