Coordination nanosheets are a unique class of two-dimensional (2D) materials that are formed by coordination bonds between planar organic ligands and metal ions. These 2D nanomaterials are increasingly utilized in energy storage, electronic devices, and as electrode-based catalysts due to their excellent electronic, optical, redox properties, and catalytic activity.
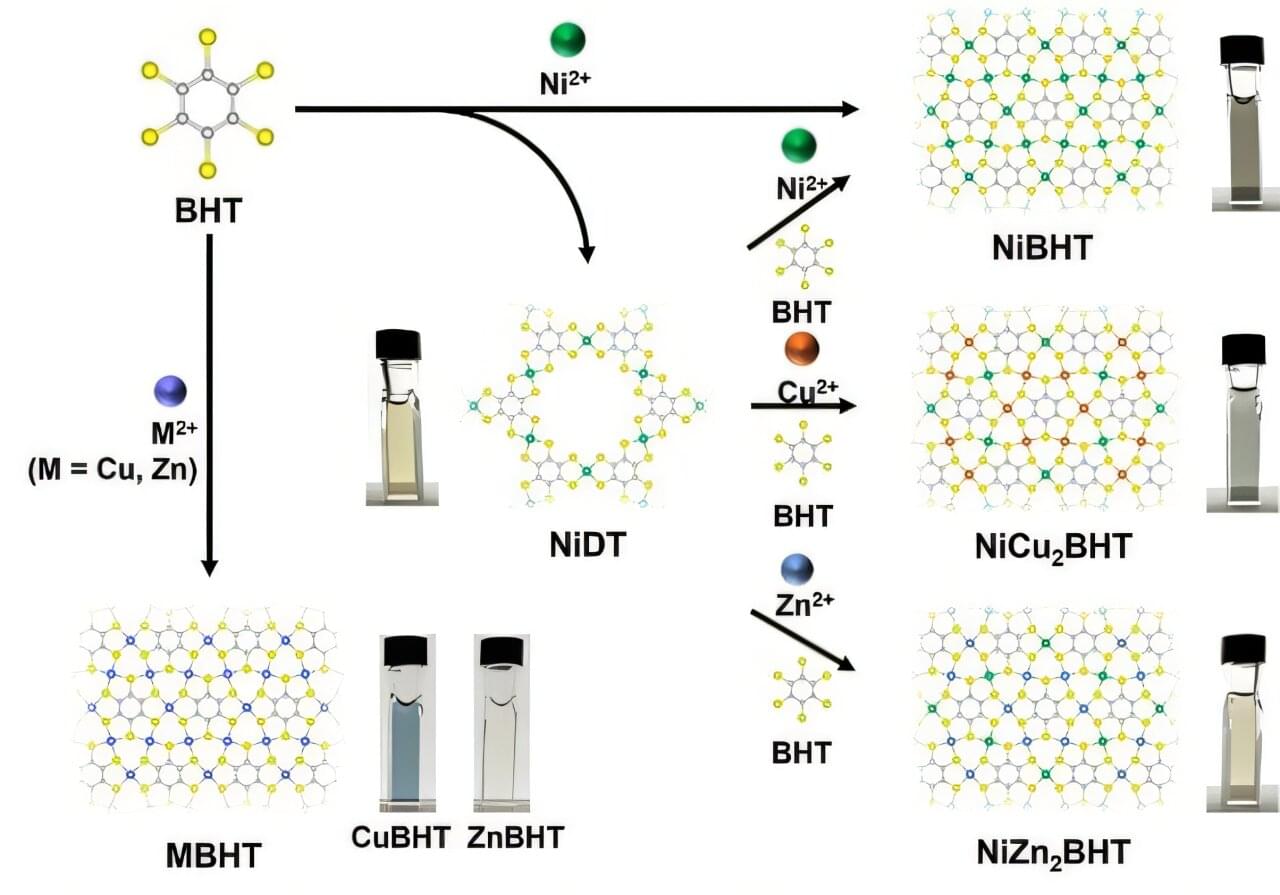
Over the last decade, coordination nanosheets composed of various transition metal ions, such as nickel (Ni) ions linked to benzenehexathiol (BHT)—an organic compound—have been successfully synthesized in laboratories. However, their production has relied on a two-phase interfacial reaction that occurs between two immiscible phases of matter.
Furthermore, the selective synthesis of well-organized heterometallic nanosheets, containing two or more metal ions, has proven to be difficult. To address these two major issues limiting the production of novel coordination nanosheets, a team of researchers led by Professor Hiroshi Nishihara, from the Research Institute for Science and Technology (RIST), Tokyo University of Science (TUS), Japan, has conducted a series of innovative experiments.