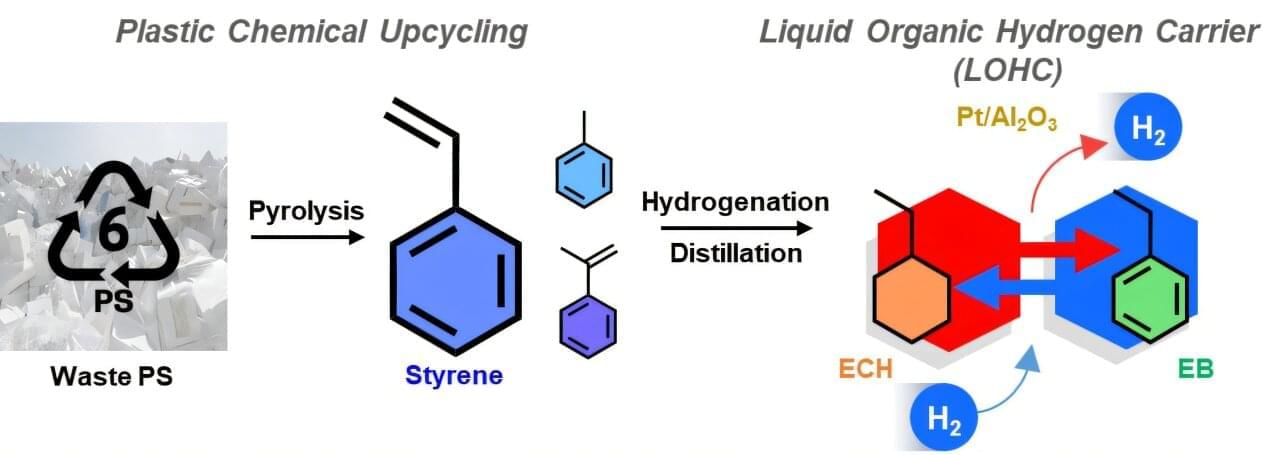
A research team affiliated with UNIST has unveiled a novel technology that enables hydrogen to be stored within polystyrene-derived materials, particularly those originating from Styrofoam. The research is published in the journal ACS Catalysis.
This advancement not only offers a solution to the low recycling rate of polystyrene —less than 1%—but also makes hydrogen storage and transportation more practical and accessible, addressing the challenges associated with handling gaseous hydrogen.
Led by Professor Kwangjin An from the School of Energy and Chemical Engineering at UNIST, in collaboration with Dr. Hyuntae Sohn from KIST and Professor Jeehoon Han from POSTECH, the team successfully designed a comprehensive, closed-loop system to convert waste polystyrene into a liquid organic hydrogen carrier (LOHC). This innovative process enables efficient hydrogen storage, retrieval, and reuse.