We now have information that this sub-250-grams DJI Mavic Mini launch date will be very soon. As soon as October 30th. No information on any possible…
Get the latest international news and world events from around the world.

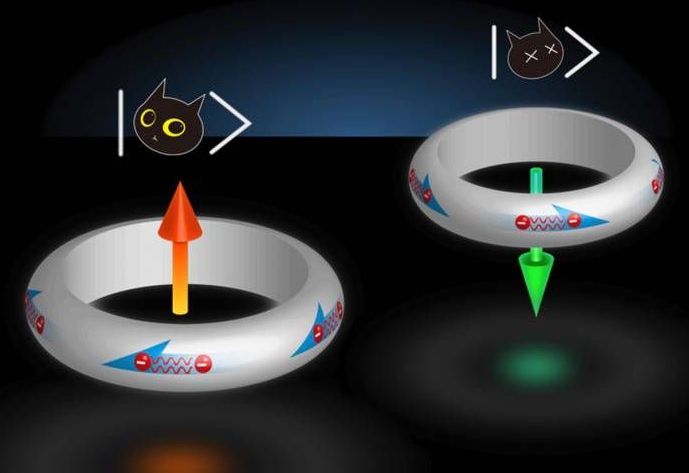
Schrodinger’s superconductor naturally stable in two states at once
Quantum computers have the potential to someday far outperform our traditional machines, thanks to their ability to store data on “qubits” that can exist in two states at once. That sounds good in theory, but in practice it’s hard to make materials that can do that and stay stable for long periods of time. Now, researchers from Johns Hopkins University have found a superconducting material that naturally stays in two states at once, which could be an important step towards quantum computers.
Our current computers are built on the binary system. That means they store and process information as binary “bits” – a series of ones and zeroes. This system has worked well for us for the better part of a century, but the general rate of computing progress has started to slow down in recent years.
Quantum computers could turn that trend on its head. The key is the use of qubits, which can store data as either a one, a zero or both at the same time – much like Schrödinger’s famous thought experiment with the cat that’s both alive and dead at the same time. Using that extra power, quantum computers would be able to outperform traditional ones at tasks involving huge amounts of data, such as AI, weather forecasting, and drug development.

Are ‘Flatliners’ Really Conscious After Death?
https://www.ncbi.nlm.nih.gov/pubmed/25301715
What happens in the brain and body in the moments after cardiac arrest?

Meet the new prototype in electromagnetic oil spill remediation technology
Many Fermilab followers are aware that Fermilab’s Office of Partnerships & Technology Transfer licensed the laboratory’s electromagnetic oil spill remediation technology to Natural Science LLC in 2015. This agreement enabled Natural Science, led by physicist and inventor Arden Warner, to design and develop a novel electromagnetic technology for cleaning oil spills. A key milestone of the agreement was to produce the first prototype and then move toward commercialization.
That prototype is now here. The concept that started as demonstrations with water, oil and magnetite in a 9-ounce cup has developed into a full-scale device. With Natural Science’s permission, we are sharing some images of this full-scale prototype below.
As you can see, the technology has come a long way from the permanent magnet demonstration videos you may have seen years ago when this was a mere concept. The system includes a scalable string of floating solenoid modules feeding a magnetic ramp and separator apparatus. Much engineering has gone into making these components work together as an oil spill solution.

Quantum state of single electrons controlled by ‘surfing’ on sound waves
Researchers have successfully used sound waves to control quantum information in a single electron, a significant step towards efficient, robust quantum computers made from semiconductors.
The international team, including researchers from the University of Cambridge, sent high-frequency sound waves across a modified semiconductor device to direct the behaviour of a single electron, with efficiencies in excess of 99 percent. The results are reported in the journal Nature Communications.
A quantum computer would be able to solve previously unsolvable computational problems by taking advantage of the strange behaviour of particles at the subatomic scale, and quantum phenomena such as entanglement and superposition. However, precisely controlling the behaviour of quantum particles is a mammoth task.
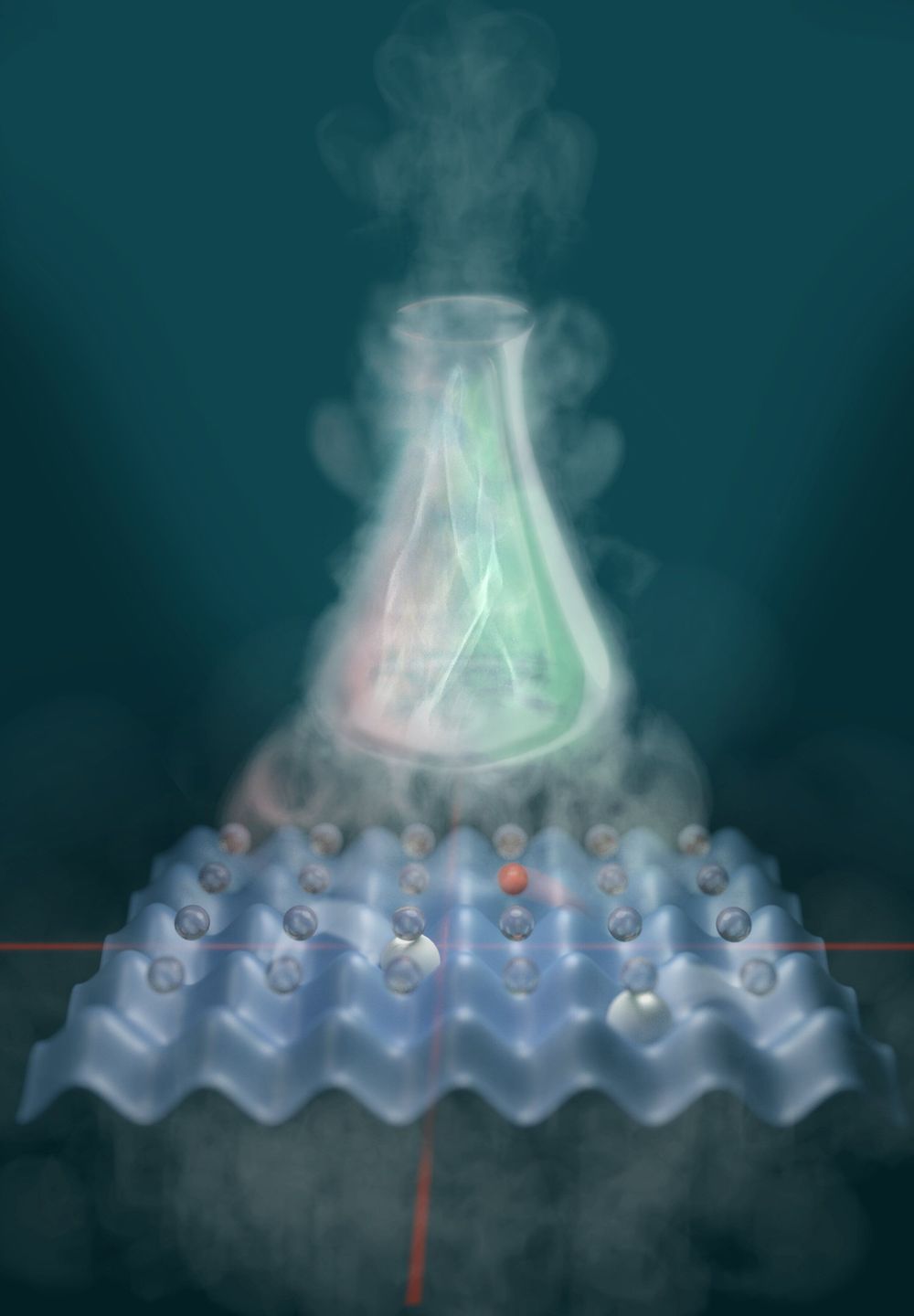
New approach for the simulation of quantum chemistry—modelling the molecular architecture
Searching for new substances and developing new techniques in the chemical industry: tasks that are often accelerated using computer simulations of molecules or reactions. But even supercomputers quickly reach their limits. Now researchers at the Max Planck Institute of Quantum Optics in Garching (MPQ) have developed an alternative, analogue approach. An international team around Javier Argüello-Luengo, Ph.D. candidate at the Institute of Photonic Sciences (ICFO), Ignacio Cirac, Director and Head of the Theory Department at the MPQ, Peter Zoller, Director at the Institute of Quantum Optics and Quantum Information in Innsbruck (IQOQI), and others have designed the first blueprint for a quantum simulator that mimics the quantum chemistry of molecules. Like an architectural model can be used to test the statics of a future building, a molecule simulator can support investigating the properties of molecules. The results are now published in the scientific journal Nature.
Using hydrogen, the simplest of all molecules, as an example, the global team of physicists from Garching, Barcelona, Madrid, Beijing and Innsbruck theoretically demonstrate that the quantum simulator can reproduce the behaviour of a real molecule’s electron shell. In their work, they also show how experimental physicists can build such a simulator step by step. “Our results offer a new approach to the investigation of phenomena appearing in quantum chemistry,” says Javier Argüello-Luengo. This is highly interesting for chemists because classical computers notoriously struggle to simulate chemical compounds, as molecules obey the laws of quantum physics. An electron in its shell, for example, can rotate to the left and right simultaneously. In a compound of many particles, such as a molecule, the number of these parallel possibilities multiplies. Because each electron interacts with each other, the complexity quickly becomes impossible to handle.
As a way out, in 1982, the American physicist Richard Feynman suggested the following: We should simulate quantum systems by reconstructing them as simplified models in the laboratory from individual atoms, which are inherently quantum, and therefore implying a parallelism of the possibilities by default. Today, quantum simulators are already in use, for example to imitate crystals. They have a regular, three-dimensional atomic lattice which is imitated by several intersecting laser beams, the “optical lattice.” The intersection points form something like wells in an egg carton into which the atoms are filled. The interaction between the atoms can be controlled by amplifying or attenuating the rays. This way researchers gain a variable model in which they can study atomic behavior very precisely.
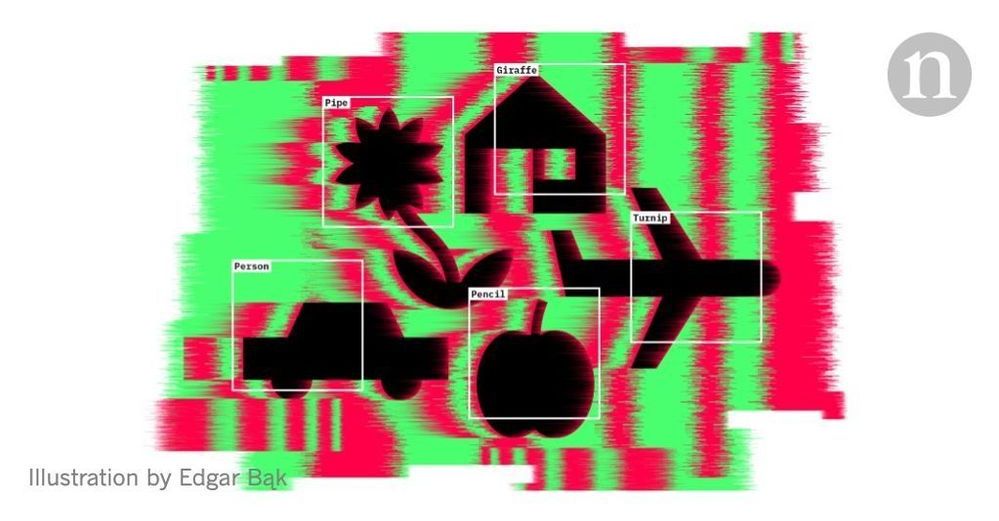
Why deep-learning AIs are so easy to fool
These are just some examples of how easy it is to break the leading pattern-recognition technology in AI, known as deep neural networks (DNNs). These have proved incredibly successful at correctly classifying all kinds of input, including images, speech and data on consumer preferences. They are part of daily life, running everything from automated telephone systems to user recommendations on the streaming service Netflix. Yet making alterations to inputs — in the form of tiny changes that are typically imperceptible to humans — can flummox the best neural networks around.
Artificial-intelligence researchers are trying to fix the flaws of neural networks.
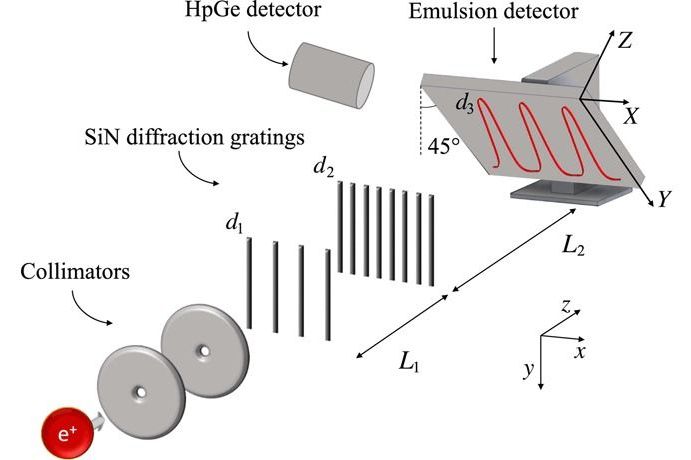
First Ever Double-Slit Experiment Performed with Antimatter
Positrons traverse two circular 2-mm-wide collimators 10.2 cm apart. The interferometer is composed of two SiN diffraction gratings with periodicity d1 and d2, respectively, separated by L1 = (118.1 ± 0.2) mm. Interference fringes with d3 periodicity are expected at L2 = (576 ± 5) mm. The emulsion is tilted so that the Y axis in the reference frame of the emulsion surface (X, Y) forms a 45° angle with the y axis of the laboratory. Gamma rays (511 keV) from positron annihilation in the emulsion are monitored with a high-purity germanium (HpGe) detector for rate measurement.
Courtesy of ScienceMag.org

82-Year-Old Woman With Dementia Gets Her Memory Back After Changing Her Diet
A message to our readers: Have you checked out our NEW on demand video platform called CETV? We created it to combat censorship and support the important journalism we are doing. Visit CETV.one to sign up for a free 7 day trial here. Thank you for your support!
Recently, an 82-year-old woman who suffered from dementia, who couldn’t recognize her own son has miraculously got her memory back after changing her diet.
When his mother’s condition became so severe that for her own safety she had to be kept in the hospital, Mark Hatzer almost came to terms with losing another parent.