A new protocol determines if quantum information has been faithfully transmitted in cases where transmission hardware may not work as intended.



In a breakthrough for antimatter research, the BASE collaboration at CERN has kept an antiproton—the antimatter counterpart of a proton—oscillating smoothly between two different quantum states for almost a minute while trapped. The achievement, reported in a paper published today in the journal Nature, marks the first demonstration of an antimatter quantum bit, or qubit, and paves the way for substantially improved comparisons between the behavior of matter and antimatter.

Using the James Webb Space Telescope (JWST), the Hubble Space Telescope (HST) and the Gemini Observatory, European astronomers have observed a nearby cold brown dwarf known as WISE 1738. Results of the observational campaign, published July 16 on the arXiv preprint server, deliver important insights into the physical properties and atmospheric chemistry of this object.
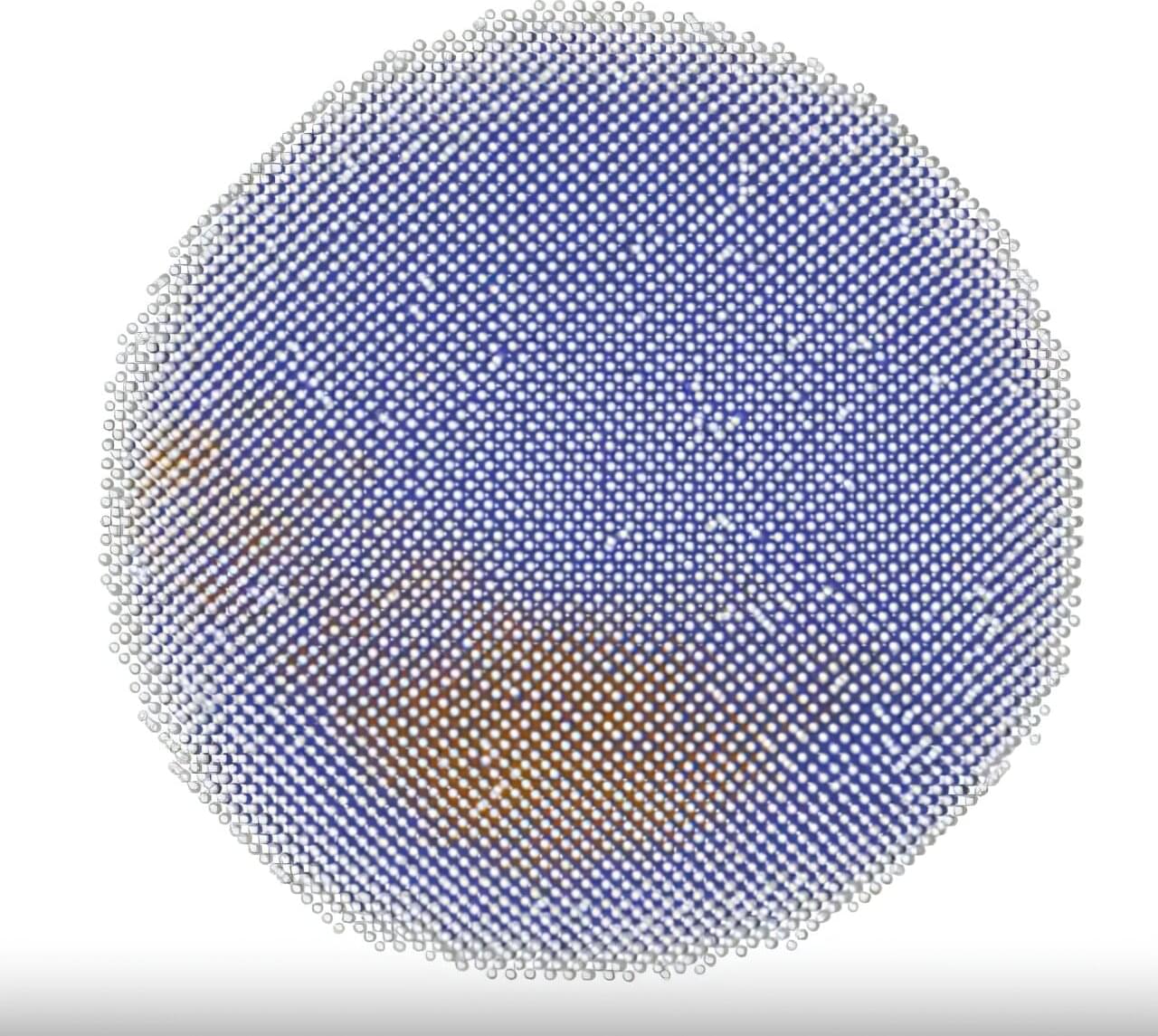
The secret to how steel hardens and shape-memory alloys snap into place lies in rapid, atomic-scale shifts that scientists have struggled to observe in materials. Now, Cornell researchers are revealing how these transformations unfold, particle by particle, through advanced modeling techniques.
Using custom-built computer simulations, Julia Dshemuchadse, assistant professor of materials science and engineering at Cornell Engineering, and Hillary Pan, Ph.D., have visualized solid-solid phase transitions in unprecedented detail, capturing the motion of every particle in a theoretical material as its crystal structure morphs into another.
Their findings, published in the Proceedings of the National Academy of Sciences, reveal not only classical transformation mechanisms, but also entirely new ones, reshaping how scientists understand this fundamental process in materials science.

What do children’s building blocks and quantum computing have in common? The answer is modularity.
It is difficult for scientists to build quantum computers monolithically—that is, as a single large unit. Quantum computing relies on the manipulation of millions of information units called qubits, but these qubits are difficult to assemble. The solution? Finding modular ways to construct quantum computers. Like plastic children’s bricks that lock together to create larger, more intricate structures, scientists can build smaller, higher-quality modules and string them together to form a comprehensive system.
Recognizing the potential of these modular systems, researchers from The Grainger College of Engineering at the University of Illinois Urbana-Champaign have presented an enhanced approach to scalable quantum computing by demonstrating a viable and high-performance modular architecture for superconducting quantum processors.

Psychology research suggests that the human body, particularly the muscles on our face, plays a key part in the processing of others’ emotions. For instance, past findings suggest that when we see another person smiling or frowning, we often unconsciously mimic their facial expression, and this helps us interpret their emotions.
Theories suggest that the mimicry of facial expressions sends signals from our facial muscles to the brain, broadly referred to as “facial feedback,” which in turn contributes to the interpretation of other people’s emotions. So far, however, the contribution of this feedback to emotion recognition and how its contribution unfolds over time remain poorly understood.
Researchers at the University of Essex recently carried out a study to investigate the effects of facial feedback on the perception of emotions at different stages of visual processing, using a technique known as facial neuromuscular electrical stimulation (fNMES). Their findings, published in Communications Psychology, suggest that signals generated by the movements of muscles on people’s faces influence how they interpret the emotions of others, particularly during the earlier stages of visual processing.
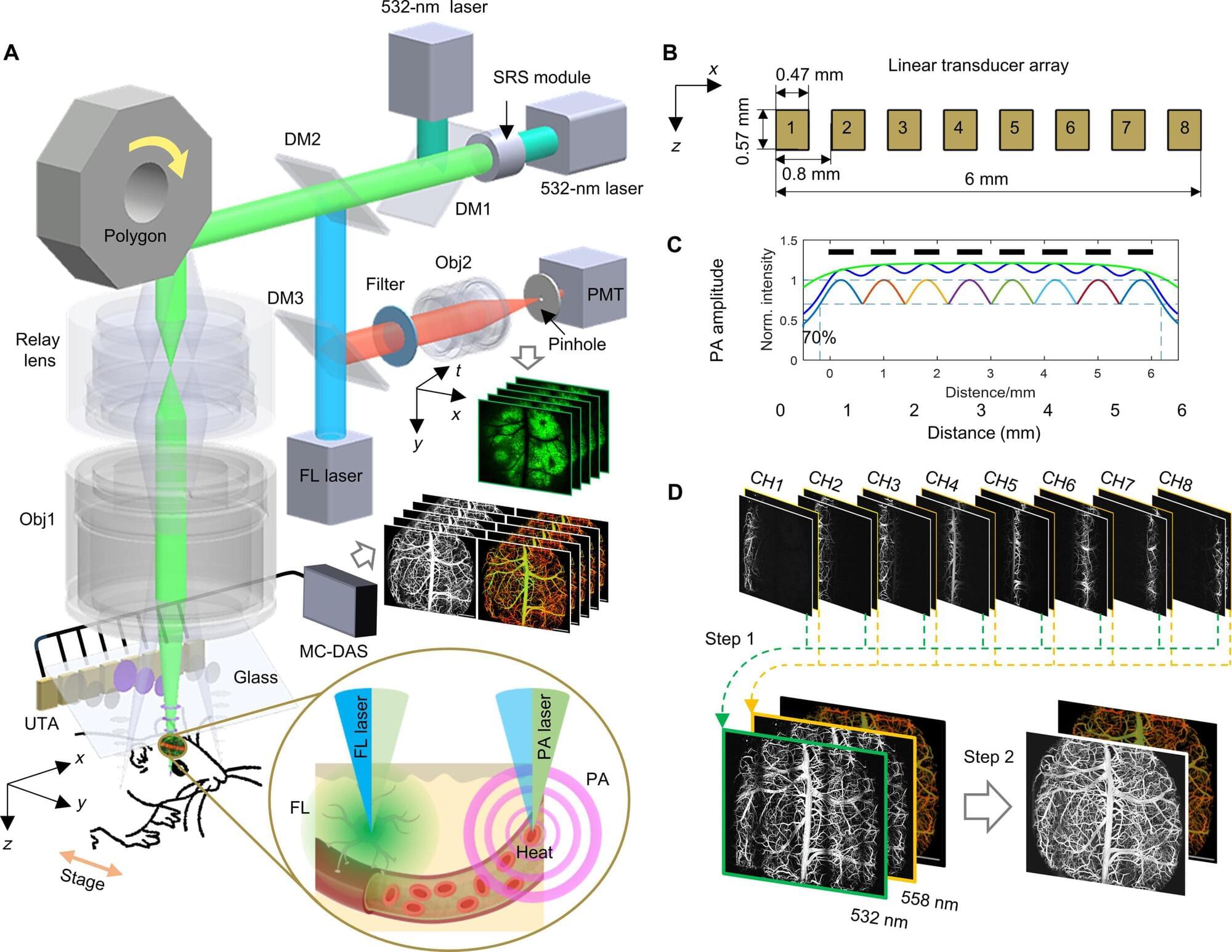

Copenhagen University Hospital’s VIRTU Research Group reports that an immersive virtual reality-assisted therapy called Challenge-VRT yielded a statistically significant, short-term reduction in auditory verbal hallucination severity among Danish adults with schizophrenia spectrum disorders.
Auditory verbal hallucinations rank among the most frequent and distressing features of schizophrenia, affecting roughly 75% of patients and resisting medication in about one-third. Approximately 13% of patients experience worsening hallucinations during their first decade of illness.
Current cognitive behavioral and relational psychotherapies show modest effects, leaving a clear unmet need for innovative treatment approaches.
Why do so many people relapse after quitting cocaine? A new study from The Hebrew University reveals that a specific “anti-reward” brain circuit becomes hyperactive during withdrawal—driving discomfort and pushing users back toward the drug. Surprisingly, this circuit may also serve as a built-in protective mechanism, offering new hope for addiction treatment.
Cocaine addiction has long been understood as a tug-of-war between reward and restraint. The rush of dopamine keeps users hooked, while withdrawal triggers anxiety, depression, and despair. But a new study by researchers at The Hebrew University of Jerusalem reveals that it’s not just the craving for pleasure—but the brain’s aversion to pain—that plays a powerful role in relapse.
Led by Prof. Yonatan M. Kupchik and Ph.D. student Liran Levi from the Faculty of Medicine, the study, appearing in Science Advances, identifies a specific “anti-reward” network deep in the brain that undergoes lasting changes during cocaine use, withdrawal, and re-exposure. This glutamatergic network, located in the ventral pallidum, is emerging as a key player in addiction—and a promising target for future therapies.
A new study of children in schooled and unschooled environments, published in Proceedings of the National Academy of Sciences, raises questions about some of the assumptions underlying the way psychologists and scholars of cognitive science think about these processes.
Instead of defining an innate, basic feature of human cognition, the executive functions supposedly captured in the assessments are likelier to depend on the influence of formal schooling.
The study, “The cultural construction of ‘executive function,’” tested children in the Kunene region of Africa, which spans the countries of Namibia and Angola, as well as children in the U.K. and Bolivia. Children in rural areas of Kunene who received limited or no formal schooling differed profoundly in so-called executive function testing from their schooled peers, or a “typical” Western schooled sample.