Francis Halzen, the lead scientist of the IceCube Neutrino Detector, explains how light sensors buried deep in the ice at the South Pole detected a neutrino that traveled four billion light-years.


Francis Halzen, the lead scientist of the IceCube Neutrino Detector, explains how light sensors buried deep in the ice at the South Pole detected a neutrino that traveled four billion light-years.
With a quantum coprocessor in the cloud, physicists from Innsbruck, Austria, open the door to the simulation of previously unsolvable problems in chemistry, materials research or high-energy physics. The research groups led by Rainer Blatt and Peter Zoller report in the journal Nature how they simulated particle physics phenomena on 20 quantum bits and how the quantum simulator self-verified the result for the first time.
Many scientists are currently working on investigating how quantum advantage can be exploited on hardware already available today. Three years ago, physicists first simulated the spontaneous formation of a pair of elementary particles with a digital quantum computer at the University of Innsbruck. Due to the error rate, however, more complex simulations would require a large number of quantum bits that are not yet available in today’s quantum computers. The analog simulation of quantum systems in a quantum computer also has narrow limits. Using a new method, researchers around Christian Kokail, Christine Maier und Rick van Bijnen at the Institute of Quantum Optics and Quantum Information (IQOQI) of the Austrian Academy of Sciences have now surpassed these limits. They use a programmable ion trap quantum computer with 20 quantum bits as a quantum coprocessor, in which quantum mechanical calculations that reach the limits of classical computers are outsourced.
LEDs are already pretty tiny, but they just got a whole lot smaller. Researchers at the University of Washington have built what they say are the “thinnest-possible LEDs” — tiny lights that measure just three atoms thick. “Such thin and foldable LEDs are critical for future portable and integrated electronic devices,” Xiaodong Xu, co-author of a paper on the research that was published over the weekend in Nature Nanotechnology, says in a statement. At three atoms thick, the researchers’ LEDs are said to be 10 to 20 times thinner than conventional LEDs, opening up a number of potential new uses for them.

Lawrence Livermore National Laboratory (LLNL) scientists in collaboration with University of Nevada Las Vegas (UNLV) have discovered a previously unknown pressure induced phase transition for TATB that can help predict detonation performance and safety of the explosive. The research appears in the May 13 online edition of the Applied Physics Letters and it is highlighted as a cover and featured article.
1,3,5-Triamino-2,4,6- trinitrobenzene (TATB), the industry standard for an insensitive high explosive, stands out as the optimum choice when safety (insensitivity) is of utmost importance. Among similar materials with comparable explosive energy release, TATB is remarkably difficult to shock-initiate and has a low friction sensitivity. The causes of this unusual behavior are hidden in the high-pressure structural evolution of TATB. Supercomputer simulations of explosives detonating, running on the world’s most powerful machines at LLNL, depend on knowing the exact locations of the atoms in the crystal structure of an explosive. Accurate knowledge of atomic arrangement under pressure is the cornerstone for predicting the detonation performance and safety of an explosive.
The team performed experiments utilizing a diamond anvil cell, which compressed TATB single crystals to a pressure of more than 25 GPa (250,000 times atmospheric pressure). According to all previous experimental and theoretical studies, it was believed that the atomic arrangement in the crystal structure of TATB remains the same under pressure. The project team challenged the consensus in the field aiming to clarify the high-pressure structural behaviour of TATB.

Given that everything at its base atom is moving maybe our interpretation of reality may be different than its actuality. From shooting photons bouncing off surfaces the world is a cacophony of all sorts of things happening at once.
A provocative new column in Scientific American floats the idea that what’s fundamentally real in the universe — its actual, base reality — isn’t the quarks, fields, and quantum phenomena that seem to comprise it.
Instead, according to scientist and philosopher Bernardo Kastrup, some are starting to suspect that matter itself is an illusion — and that the only real thing is information.
Information Space
The basic idea is that the physical universe exists because we perceive it — it’s a sort of mass hallucination we use to make sense of the mathematical relationships of objects.
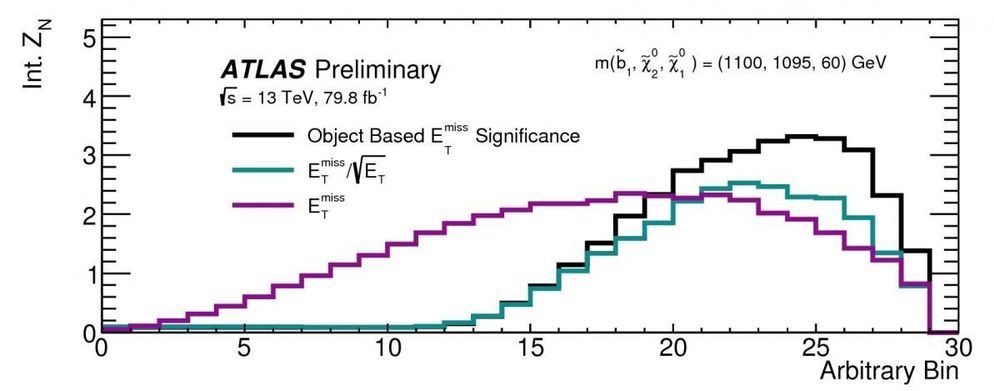
Dark matter is an unknown type of matter present in the universe that could be of particle origin. One of the most complete theoretical frameworks that includes a dark matter candidate is supersymmetry. Many supersymmetric models predict the existence of a new stable, invisible particle called the lightest supersymmetric particle (LSP), which has the right properties to be a dark matter particle.
The ATLAS Collaboration at CERN has recently reported two new results on searches for an LSP that exploited the experiment’s full Run 2 data sample taken at 13 TeV proton-proton collision energy. The analyses looked for the pair production of two heavy supersymmetric particles, each of which decays to observable Standard Model particles and an LSP in the detector.

The results are fascinating and spur the imagination, but don’t start investing in flux capacitors yet. This experiment also shows us that sending even a simulated particle back in time requires serious outside manipulation. To create such an external force to manipulate even one physical particle’s quantum waves is well beyond our abilities.
“We demonstrate that time-reversing even ONE quantum particle is an unsurmountable task for nature alone,” study author Vinokur wrote to the New York Times in an email [emphasis original]. “The system comprising two particles is even more irreversible, let alone the eggs — comprising billions of particles — we break to prepare an omelet.”
A press release from the Department of Energy notes that for the “timeline required for [an external force] to spontaneously appear and properly manipulate the quantum waves” to appear in nature and unscramble an egg “would extend longer than that of the universe itself.” In other words, this technology remains bound to quantum computation. Subatomic spas that literally turn back the clock aren’t happening.

A new theory proposes that faster-than-light particles known as tachyons could answer a lot of questions about the universe, writes Robyn Arianrhod.