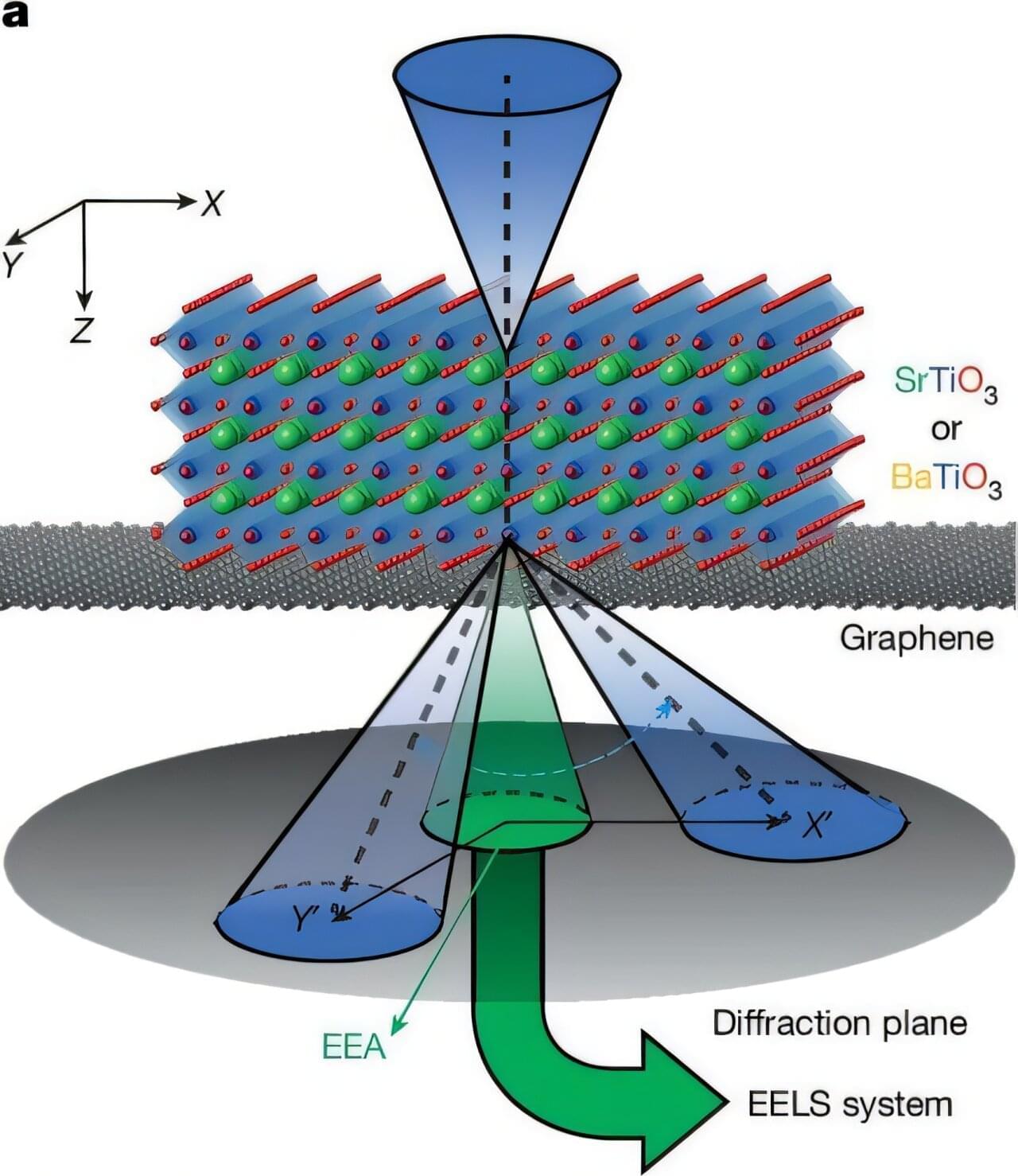
Recent research has found a new way to make graphene that adds structural defects to improve the performance of the material that could have benefits across a range of applications—from sensors and batteries, to electronics.
Scientists from the University of Nottingham’s School of Chemistry, University of Warwick and Diamond Light Source developed a single-step process to grow graphene-like films using a molecule, Azupyrene, whose shape mimics that of the desired defect. The research has been published today in Chemical Science.
David Duncan, Associate Professor at the University of Nottingham and one of the study’s lead authors, explains, “Our study explores a new way to make graphene, this super-thin, super-strong material is made of carbon atoms, and while perfect graphene is remarkable, it is sometimes too perfect. It interacts weakly with other materials and lacks crucial electronic properties required in the semiconductor industry.”