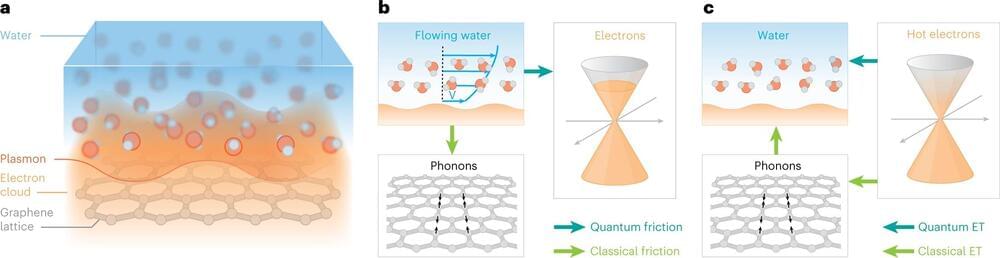
Water and carbon make a quantum couple: the flow of water on a carbon surface is governed by an unusual phenomenon dubbed quantum friction. A new work published in Nature Nanotechnology experimentally demonstrates this phenomenon—which was predicted in a previous theoretical study—at the interface between liquid water and graphene, a single layer of carbon atoms. Advanced ultrafast techniques were used to perform this study. These results could lead to applications in water purification and desalination processes and maybe even to liquid-based computers.
For the last 20 years, scientists have been puzzled by how water behaves near carbon surfaces. It may flow much faster than expected from conventional flow theories or form strange arrangements such as square ice. Now, an international team of researchers from the Max Plank Institute for Polymer Research of Mainz (Germany), the Catalan Institute of Nanoscience and Nanotechnology (ICN2, Spain), and the University of Manchester (England), reports in the study published in Nature Nanotechnology on June 22, 2023, that water can interact directly with the carbon’s electrons—a quantum phenomenon that is very unusual in fluid dynamics.
A liquid, such as water, is made up of small molecules that randomly move and constantly collide with each other. A solid, in contrast, is made of neatly arranged atoms that bathe in a cloud of electrons. The solid and the liquid worlds are assumed to interact only through collisions of the liquid molecules with the solid’s atoms—the liquid molecules do not “see” the solid’s electrons. Nevertheless, just over a year ago, a paradigm-shifting theoretical study proposed that at the water-carbon interface, the liquid’s molecules and the solid’s electrons push and pull on each other, slowing down the liquid flow: this new effect was called quantum friction. However, the theoretical proposal lacked experimental verification.