Wirelessly powered microchips, which have an ~1 GHz electromagnetic transcutaneous link to an external telecom hub, can be used for multichannel in vivo neural sensing, stimulation and data acquisition.



Yes this says a 3 year epigenetic clock reversal in just 8 weeks thanks to diet and lifestyle changes. There is a list of supplements too:
Alpha ketoglutarate, vitamin C and vitamin A curcumin, epigallocatechin gallate (EGCG), rosmarinic acid, quercetin, luteolin.
Manipulations to slow biological aging and extend healthspan are of interest given the societal and healthcare costs of our aging population. Herein we report on a randomized controlled clinical trial conducted among 43 healthy adult males between the ages of 50–72. The 8-week treatment program included diet, sleep, exercise and relaxation guidance, and supplemental probiotics and phytonutrients. The control group received no intervention. Genome-wide DNA methylation analysis was conducted on saliva samples using the Illumina Methylation Epic Array and DNAmAge was calculated using the online Horvath DNAmAge clock (2013). The diet and lifestyle treatment was associated with a 3.23 years decrease in DNAmAge compared with controls (p=0.018). DNAmAge of those in the treatment group decreased by an average 1.96 years by the end of the program compared to the same individuals at the beginning with a strong trend towards significance (p=0.066). Changes in blood biomarkers were significant for mean serum 5-methyltetrahydrofolate (+15%, p=0.004) and mean triglycerides (−25%, p=0.009). To our knowledge, this is the first randomized controlled study to suggest that specific diet and lifestyle interventions may reverse Horvath DNAmAge (2013) epigenetic aging in healthy adult males. Larger-scale and longer duration clinical trials are needed to confirm these findings, as well as investigation in other human populations.
Keywords: DNA methylation, epigenetic, aging, lifestyle, biological clock.
Advanced age is the largest risk factor for impaired mental and physical function and many non-communicable diseases including cancer, neurodegeneration, type 2 diabetes, and cardiovascular disease [1, 2]. The growing health-related economic and social challenges of our rapidly aging population are well recognized and affect individuals, their families, health systems and economies. Considering economics alone, delaying aging by 2.2 years (with associated extension of healthspan) could save $7 trillion over fifty years [3]. This broad approach was identified to be a much better investment than disease-specific spending. Thus, if interventions can be identified that extend healthspan even modestly, benefits for public health and healthcare economics will be substantial.
The Future of Everything covers the innovation and technology transforming the way we live, work and play, with monthly issues on health, money, cities and more. This month is Education & Learning, online starting Aug. 6 and in the paper on Aug. 13.
No one has yet deciphered the brain signals that encode a complex thought, turn an idea into words or make a lasting memory. But powerful clues are emerging to drive the neurotechnology of learning, scientists say.
On the frontier of neuroscience, researchers are inventing devices to enhance learning abilities, from wearable nerve stimulators that boost mental focus to headsets for wireless brain-to-brain communication.
Is a unique app designed to put you in the shoes of someone living with dementia. See one of the 360 clips from the experience.
http://awalkthroughdementia.org/
https://itunes.apple.com/us/app/a-walk-through-dementia/id1242267344
https://play.google.com/store/apps/details?id=com.alzheimers…a&hl=en_GB
A 21st Century Mystery School — “Creating New Paradigms In Wellness And Wisdom Never Seen Before, And Never More Needed Than Now” — Dr. Dennis McKenna, Founder, McKenna Academy of Natural Philosophy.
Dr. Dennis McKenna is an American ethnopharmacologist, research pharmacognosist, lecturer, author, and Founder of the McKenna Academy of Natural Philosophy (www.mckenna.academy).
Dr. McKenna is a founding board member and the director of ethnopharmacology at the Heffter Research Institute, a non-profit organization concerned with the investigation of the potential therapeutic uses of psychedelic medicines. He also serves on the Advisory Board of the American Botanical Council; as Founder and Executive Director for the Institute for Natural Products Research; as an Independent Research Consultant to the Phytomedicine and Nutraceutical Industry; was formerly on the Editorial Board of Phytomedicine, International Journal of Phytotherapy and Phytopharmacology; and is an adjunct professor in the Center for Spirituality and Healing at the University of Minnesota.
Dr. McKenna received his Master’s Degree in Botany from the University of Hawaii in 1,979 his Ph.D. in Botanical Sciences from the University of British Columbia in 1,984 and continued into post-doctoral research fellowships in the Laboratory of Clinical Pharmacology, National Institute of Mental Health (NIMH), and in the Department of Neurology, Stanford University School of Medicine.
Dr. McKenna’s research led to the development of natural products for the Aveda Corporation, as well as greater awareness of natural products and medicines. He has authored or co-authored over 50 peer-reviewed scientific papers and written multiple books, including “The Brotherhood of the Screaming Abyss: My Life with Terence McKenna”, co-author of “The Invisible Landscape” with his brother Terence, and co-author of a widely recognized reference work on herbal medicines, titled “Botanical Medicines: the Desk Reference for Major Herbal Supplements”.
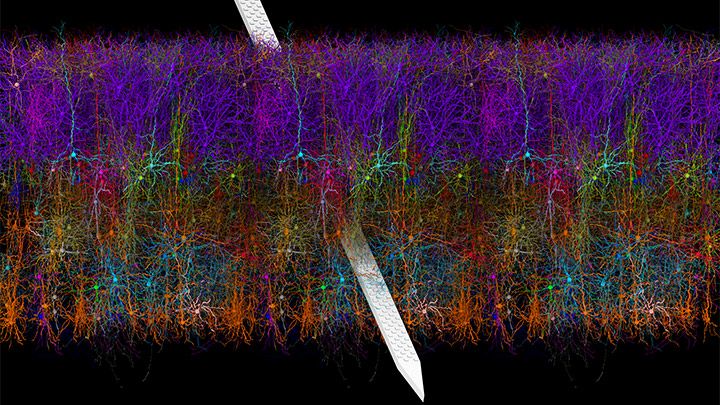
The NIH-led Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative continues to teach us about the world’s most sophisticated computer: the human brain. This striking image offers a spectacular case in point, thanks to a new tool called Visual Neuronal Dynamics (VND).
VND is not a camera. It is a powerful software program that can display, animate, and analyze models of neurons and their connections, or networks, using 3D graphics. What you’re seeing in this colorful image is a strip of mouse primary visual cortex, the area in the brain where incoming sensory information gets processed into vision.
This strip contains more than 230,000 neurons of 17 different cell types. Long and spindly excitatory neurons that point upward (purple, blue, red, orange) are intermingled with short and stubby inhibitory neurons (green, cyan, magenta). Slicing through the neuronal landscape is a neuropixels probe (silver): a tiny flexible silicon detector that can record brain activity in awake animals [1].

Pear-shaped people, whose weight is generally distributed more evenly, rather than “apple shaped” individuals with fat clustered around their middle and often around internal organs like the liver in the abdominal cavity, are considered less at risk for cardiometabolic problems like heart disease and diabetes, as well as cognitive decline, says Stranahan, neuroscientist at the Medical College of Georgia at Augusta University.
Summary: Adipocytes, or beige fat cells, are indispensable to the anti-inflammatory and neuroprotective effects of subcutaneous fat, researchers say.
Source: Medical College of Georgia at Augusta University
Beige is considered a calming paint color, and scientists have new evidence that beige fat has a similar impact on the brain, bringing down the inflammation associated with the more common white fat and providing protection from dementia.
They have found that beige fat cells, which are typically intermingled with white fat cells in the subcutaneous fat present on “pear shaped” people, mediate subcutaneous fat’s brain protection, Dr. Alexis M. Stranahan and her colleagues report in the journal Nature Communications.


The ones Teresa is handling in this Cambridge laboratory are mini bile ducts, thin tubes that carry bile from the liver to the small intestine to help with digestion.
Teresa also has gut organoids in the incubator, while down the corridor a different team is developing brain organoids.
In fact, around the world, miniatures of everything from lungs to kidneys are being coaxed gently to life. And because they function just as organs do, they are perfect for research.