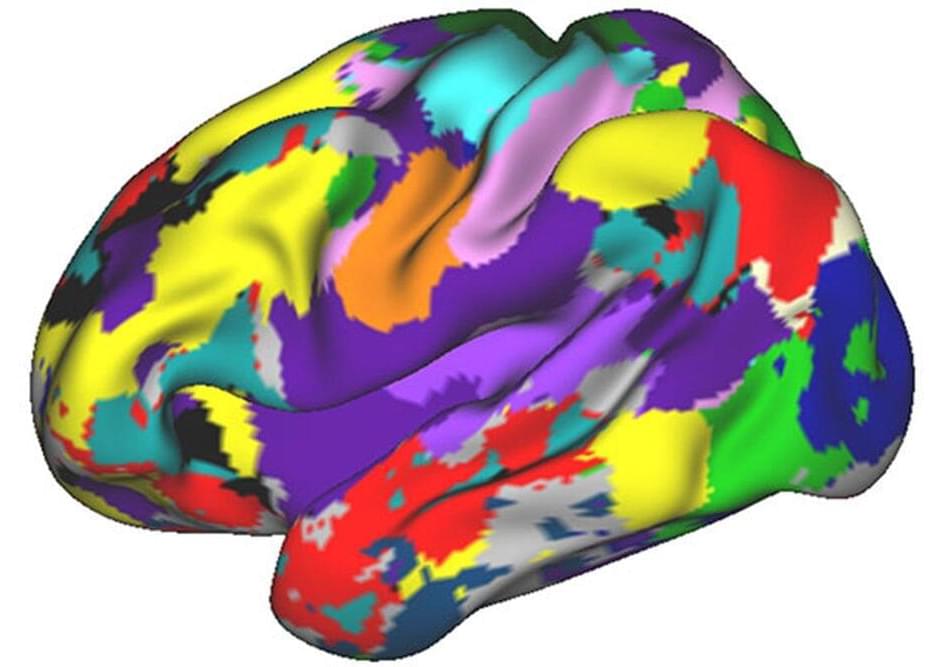
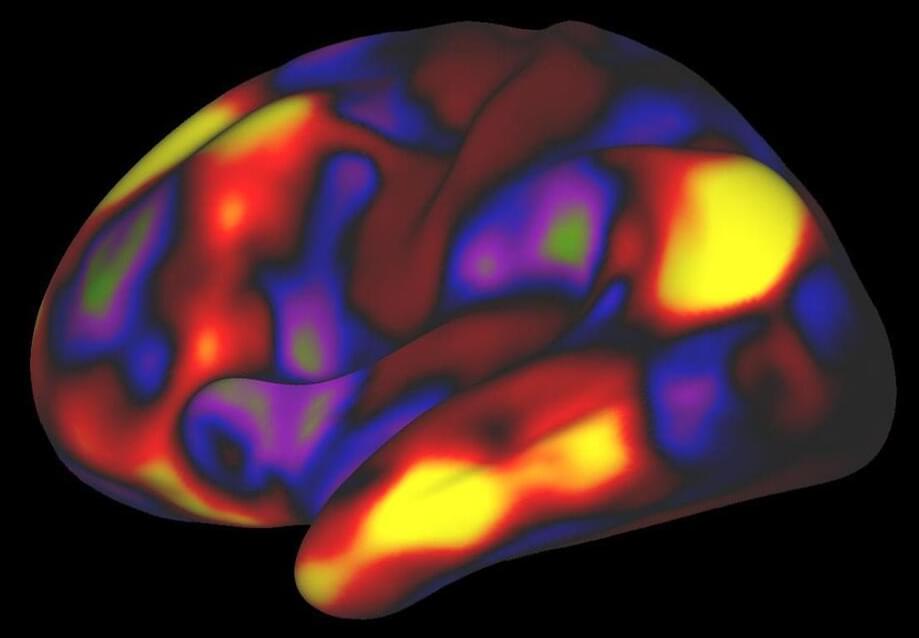
A quest to analyze the unique features of individual human brains evolved into the so-called Midnight Scan Club, a group of scientists who had big ideas but almost no funding and little time to research the trillions of neural connections that activate the body’s most powerful organ.
The research group started in 2013 by two neuroscientists at Washington University School of Medicine in St. Louis who aimed to collect a massive amount of data on individual brains. The study’s subjects were the scientists themselves and eight others, all junior faculty or graduate students.
Most efforts to analyze connections involve scanning many brains and averaging the data across groups of people. For this study, the researchers used brain-imaging techniques to evaluate brain networks that control speech and motor function, among other activities. The researchers examined individuals while resting and performing cognitive tasks such as reading.