The mathematician shares his latest theories on quantum consciousness, the structure of the universe and how to communicate with civilisations from other cosmological aeons.



The stadiums include a quiet room for those with cognitive disabilities. The FIFA World Cup to be held in Qatar this year is leading the way in accessibility for specially-abled people, according to a report by Euronews published on Wednesday. The event is making sure all can enjoy it.
Qatar unveiled a massive 40,000-seat arena for the World Cup that’s entirely crafted from shipping containers.
Built with the notion of sustainable construction in mind, Qatar officials are partnering with architects Fenwick Iribarren, Schlaich Bergermann Partner and Hilson Moran on the Ras Abu Aboud Stadium. The building could even earn a four-star rating from the Global Sustainability Assessment System (GSAS) certification.
Mental health practitioners and meditation gurus have long credited intentional breathing with the ability to induce inner calm, but scientists do not fully understand how the brain is involved in the process. Using functional magnetic resonance imaging (fMRI) and electrophysiology, researchers in the Penn State College of Engineering identified a potential link between respiration and neural activity changes in rats.
Their results were made available online ahead of publication in eLife. The researchers used simultaneous multi-modal techniques to clear the noise typically associated with brain imaging and pinpoint where breathing regulated neural activity.
“There are roughly a million papers published on fMRI—a non-invasive imaging technique that allows researchers to examine brain activity in real time,” said Nanyin Zhang, founding director of the Penn State Center for Neurotechnology in Mental Health Research and professor of biomedical engineering.

Cheers!
𝐁𝐞𝐞𝐫 𝐈𝐧𝐠𝐫𝐞𝐝𝐢𝐞𝐧𝐭 𝐌𝐚𝐲 𝐈𝐧𝐡𝐢𝐛𝐢𝐭 𝐂𝐥𝐮𝐦𝐩𝐢𝐧𝐠 𝐨𝐟 𝐀𝐥𝐳𝐡𝐞𝐢𝐦𝐞𝐫’𝐬 𝐏𝐫𝐨𝐭𝐞𝐢𝐧
𝘽𝙚𝙚𝙧 𝙞𝙨 𝙤𝙣𝙚 𝙤𝙛 𝙩𝙝𝙚 𝙤𝙡𝙙𝙚𝙨𝙩 𝙖𝙣𝙙 𝙢𝙤𝙨𝙩 𝙥𝙤𝙥𝙪𝙡𝙖𝙧 𝙗𝙚𝙫𝙚𝙧𝙖𝙜𝙚𝙨 𝙞𝙣 𝙩𝙝𝙚 𝙬𝙤𝙧𝙡𝙙, 𝙬𝙞𝙩𝙝 𝙨𝙤𝙢𝙚 𝙥𝙚𝙤𝙥𝙡𝙚 𝙡𝙤𝙫𝙞𝙣𝙜 𝙖𝙣𝙙 𝙤𝙩𝙝𝙚𝙧𝙨 𝙝𝙖𝙩𝙞𝙣𝙜 𝙩𝙝𝙚 𝙙𝙞𝙨𝙩𝙞𝙣𝙘𝙩, 𝙗𝙞𝙩𝙩𝙚𝙧 𝙩𝙖𝙨𝙩𝙚 𝙤𝙛 𝙩𝙝𝙚 𝙝𝙤𝙥𝙨 𝙪𝙨𝙚𝙙 𝙩𝙤 𝙛𝙡𝙖𝙫𝙤𝙧 𝙞𝙩𝙨 𝙢𝙖𝙣𝙮 𝙫𝙖𝙧𝙞𝙚𝙩𝙞𝙚𝙨. 𝘽𝙪𝙩 𝙖𝙣 𝙚𝙨𝙥𝙚𝙘𝙞𝙖𝙡𝙡𝙮 “𝙝𝙤𝙥𝙥𝙮” 𝙗𝙧𝙚𝙬 𝙢𝙞𝙜𝙝𝙩 𝙝𝙖𝙫𝙚 𝙪𝙣𝙞𝙦𝙪𝙚 𝙝𝙚𝙖𝙡𝙩𝙝 𝙗𝙚𝙣𝙚𝙛𝙞𝙩𝙨. 𝙍𝙚𝙘𝙚𝙣𝙩 𝙧𝙚𝙨𝙚𝙖𝙧𝙘𝙝 𝙥𝙪𝙗𝙡𝙞𝙨𝙝𝙚𝙙 𝙞𝙣 𝘼𝘾𝙎 𝘾𝙝𝙚𝙢𝙞𝙘𝙖𝙡 𝙉𝙚𝙪𝙧𝙤𝙨𝙘𝙞𝙚𝙣𝙘𝙚 𝙧𝙚𝙥𝙤𝙧𝙩𝙨 𝙩𝙝𝙖𝙩 𝙘𝙝𝙚𝙢𝙞𝙘𝙖𝙡𝙨 𝙚𝙭𝙩𝙧𝙖𝙘𝙩𝙚𝙙 𝙛𝙧𝙤𝙢 𝙝𝙤𝙥 𝙛𝙡𝙤𝙬𝙚𝙧𝙨 𝙘𝙖𝙣, 𝙞𝙣 𝙡𝙖𝙗 𝙙𝙞𝙨𝙝𝙚𝙨, 𝙞𝙣𝙝𝙞𝙗𝙞𝙩 𝙩𝙝𝙚 𝙘𝙡𝙪𝙢𝙥𝙞𝙣𝙜 𝙤𝙛 𝙖𝙢𝙮𝙡𝙤𝙞𝙙 𝙗𝙚𝙩𝙖 𝙥𝙧𝙤𝙩𝙚𝙞𝙣𝙨, 𝙬𝙝𝙞𝙘𝙝 𝙞𝙨 𝙖𝙨𝙨𝙤𝙘𝙞𝙖𝙩𝙚𝙙 𝙬𝙞𝙩𝙝 𝘼𝙡𝙯𝙝𝙚𝙞𝙢𝙚𝙧’𝙨 𝙙𝙞𝙨𝙚𝙖𝙨𝙚 (𝘼𝘿).
Beer is one of the oldest and most popular beverages in the world, with some people loving and others hating the distinct, bitter taste of the hops used to flavor its many varieties. But an especially “hoppy” brew might have unique health benefits. Recent research published in ACS Chemical Neuroscience reports that chemicals extracted from hop flowers can, in lab dishes, inhibit the clumping of amyloid beta proteins, which is associated with Alzheimer’s disease (AD).
AD is a debilitating neurodegenerative disease, often marked by memory loss and personality changes in older adults. Part of the difficulty in treating the disease is the time lag between the start of underlying biochemical processes and the onset of symptoms, with several years separating them. This means that irreversible damage to the nervous system occurs before one even realizes they may have the disease. Accordingly, preventative strategies and therapeutics that can intervene before symptoms appear are of increasing interest.
One of these strategies involves “nutraceuticals,” or foods that have some type of medicinal or nutritional function. The hop flowers used to flavor beers have been explored as one of these potential nutraceuticals, with previous studies suggesting that the plant could interfere with the accumulation of amyloid beta proteins associated with AD. So, Cristina Airoldi, Alessandro Palmioli and colleagues wanted to investigate which chemical compounds in hops had this effect.
17:12 minutes.
After decades of research using sophisticated brain imaging, there’s a growing consensus among neuroscientists that understanding the connections between brain regions may be even more important than the functions of the regions themselves. When it comes to understanding human cognition, the whole is greater than the sum of its parts.
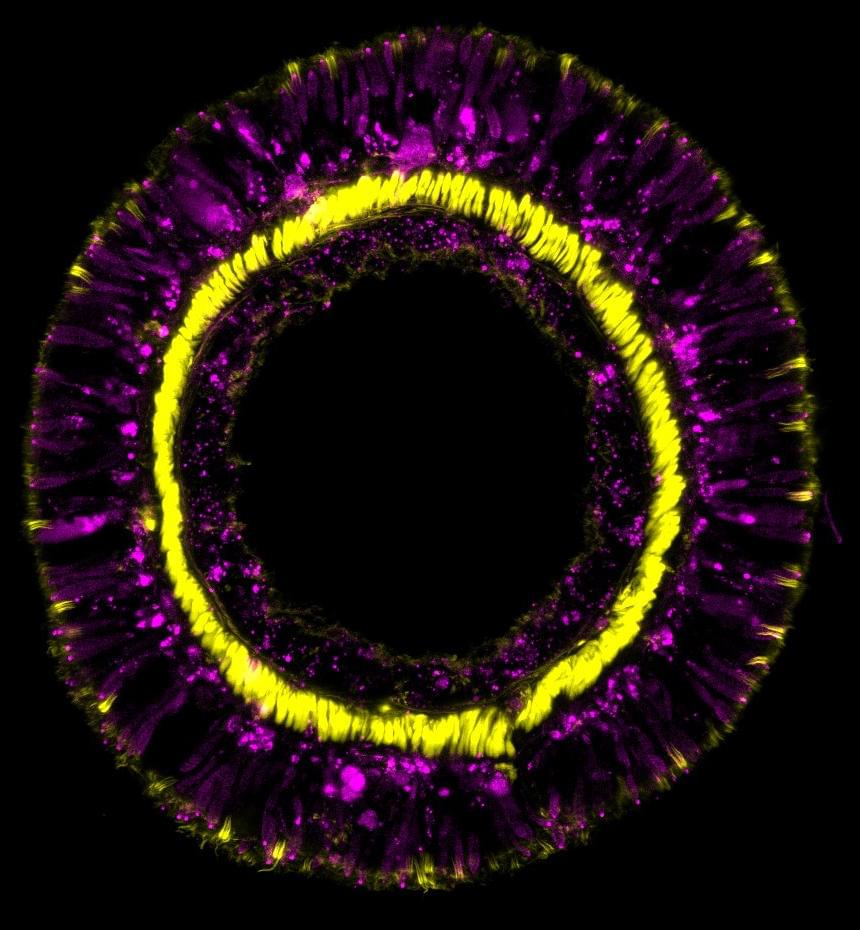
Sea anemones are seemingly immortal animals. They seem to be immune to aging and the negative impacts that humans experience over time. However, the exact reasons for their eternal youth are not completely understood.
The genetic fingerprint of the sea anemone Nematostella vectensis reveals that members of this incredibly ancient animal phylum employ the same gene cascades for neural cell differentiation as more complex organisms. These genes are also in charge of maintaining the balance of all cells in the organism during the anemone’s lifetime. These findings were recently published in the journal Cell Reports by a group of developmental biologists headed by Ulrich Technau of the University of Vienna.
Almost all animal organisms are made up of millions, if not billions, of cells that join together in intricate ways to create specific tissues and organs, which are made up of a range of cell types, such as a variety of neurons and gland cells. However, it is unclear how this critical balance of diverse cell types emerges, how it is regulated, and if the different cell types of different animal organisms have a common origin.
