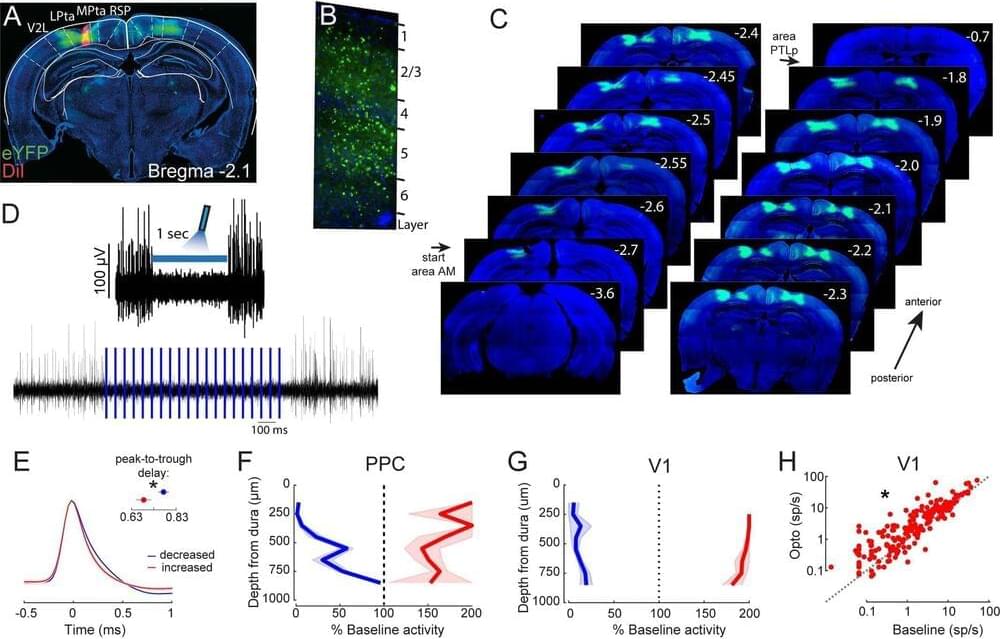
Category: neuroscience – Page 624


A Neural Net Hooked Up to a Monkey Brain Spat Out Bizarre Images
“If cells are dreaming, [these images] are what the cells are dreaming about,” neuroscientist Carlos Ponce told The Atlantic. “It exposes the visual vocabulary of the brain, in a way that’s unbiased by our anthropomorphic perspective.”
Some neurons responded to images that vaguely resembled objects that the scientists recognized, suggesting that the researchers identified the specific neurons that corresponded with particular real-world objects. A blur that resembled a monkey’s face accompanied by a red blotch may have corresponded to another monkey in the lab that wore a red collar. Another blur that resembled a human wearing a surgical mask may have represented the woman who took care of and fed the lab’s monkeys, who wore a similar mask.
Other images that the monkey neurons responded to the most were less realistic, instead taking the form of various streaks and splotches of color, according to The Atlantic.
Interview w/ Prof. Lee Smolin on Parkinson’s and getting a Deep Brain Stimulator implanted yesterday
Just yesterday (2022−08−24) Prof. Smolin had a deep brain stimulator inserted into his left Globus Pallidus to help him better manage his symptoms of Parkinson’s Disease. Here he talks about that day, his theory of PD and the journey he had been on to getting DBS.
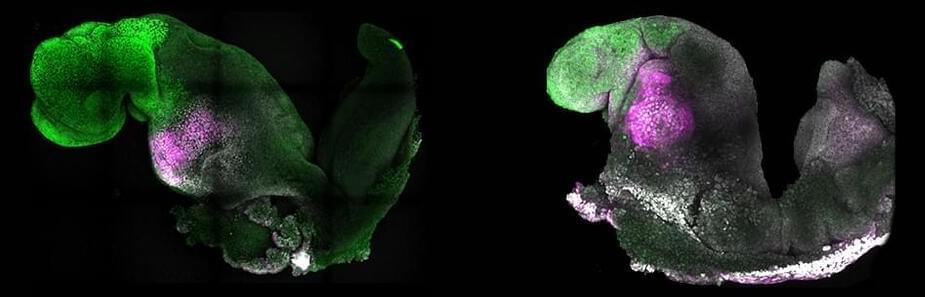
Scientists Grew a Synthetic Mouse Embryo With a Brain And a Beating Heart
Eavesdropping on the earliest conversations between tissues in an emerging life could tell us a lot about organ growth, fertility, and disease in general. It could help prevent early miscarriages, or even tell us how to grow whole replacement organs from scratch.
In a monumental leap in stem cell research, an experiment led by researchers from the University of Cambridge in the UK has developed a living model of a mouse embryo complete with fluttering heart tissues and the beginnings of a brain.
The research advances the recent success of a team comprised of some of the same scientists who pushed the limits on mimicking the embryonic development of mice using stem cells that had never seen the inside of a mouse womb.
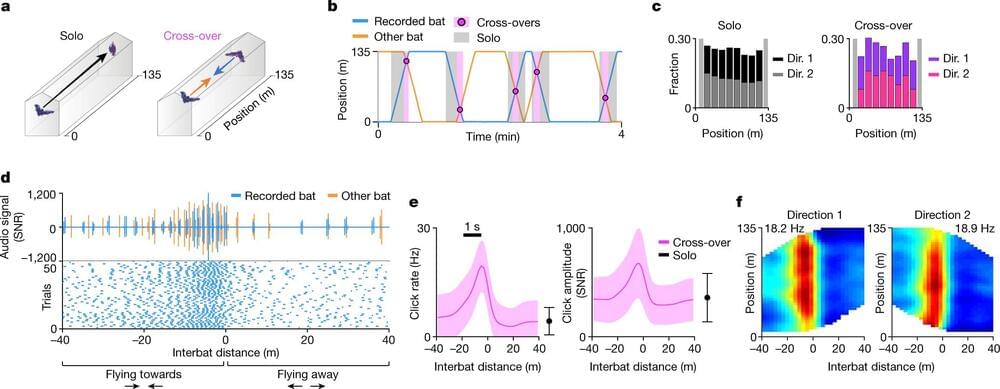
How brain circuits switch between different behaviors
Even during such routine tasks as a daily stroll, our brain sometimes needs to shift gears, switching from navigating the city to jumping out of the way of a bike or to crossing the street to greet a friend. These switches pose a challenge: How do the brain’s circuits deal with such dynamic and abrupt changes in behavior? A Weizmann Institute of Science study on bats, published today in Nature, suggests an answer that does not fit the classical thinking about brain function.
“Most brain research projects focus on one type of behavior at a time, so little is known about the way the brain handles dynamically changing behavioral needs,” says Prof. Nachum Ulanovsky of Weizmann’s Brain Sciences Department. In the new study, he and his team designed an experimental setup that mimicked real-life situations in which animals or humans rapidly switch from one behavior to another—for example, from navigation to avoiding a predator or a car crash. Graduate students Dr. Ayelet Sarel, Shaked Palgi and Dan Blum led the study, in collaboration with postdoctoral fellow Dr. Johnatan Aljadeff. The study was supervised by Ulanovsky together with Associate Staff Scientist Dr. Liora Las.
Using miniature wireless recording devices, the researchers monitored neurons in the brains of pairs of bats that had to avoid colliding with one another while flying toward each other along a 135-meter-long tunnel at the high speed of 7 meters per second. This amounted to a relative speed—that is, the rate at which the distance between the bats closed, or the sum of both bats’ speeds—of 14 meters per second, or about 50 kilometers an hour.
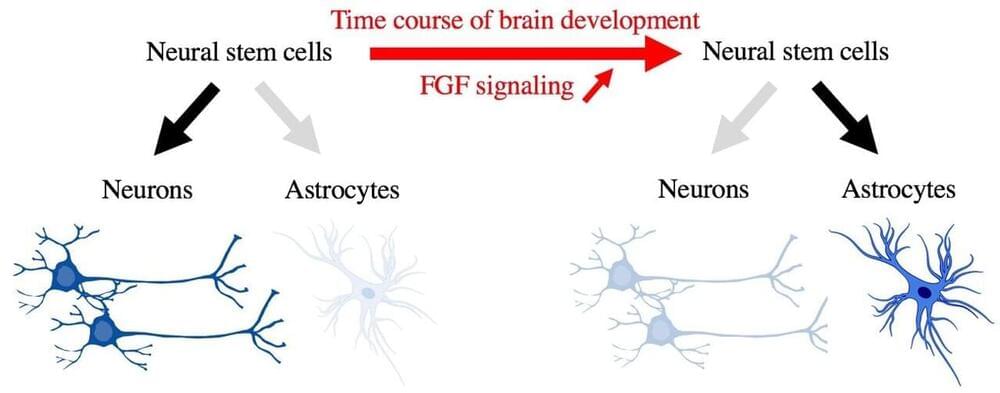
Discovery of the cell fate switch from neurons to astrocytes in the developing brain
During mammalian brain development, neural precursor cells first generate neurons and later astrocytes. This cell fate change is a key process generating proper numbers of neurons and astrocytes. Here we discovered that FGF regulates the cell fate switch from neurons to astrocytes in the developing cerebral cortex using mice. FGF is a critical extracellular regulator of the cell fate switch, necessary and sufficient, in the mammalian cerebral cortex.
Neurons and astrocytes are prominent cell types in the cerebral cortex. Neurons are the primary information processing cells in the brain, whereas astrocytes support and modulate their functions. For sound functioning of the brain, it is crucial that proper numbers of neurons and astrocytes are generated during fetal brain development. The brain could not function correctly if only neurons or astrocytes were generated.
During fetal brain development, both neurons and astrocytes are generated from neural stem cells, which give rise to almost all cells in the cerebral cortex (Figure 1). One of the characteristics of this developmental process is that neural stem cells first generate neurons and, after that, start generating astrocytes (Figure 1). The “switch” to change the cell differentiation fate of neural stem cells from neurons to astrocytes has attracted much attention, since the cell fate switch is key to the generation of proper numbers of neurons and astrocytes. However, it remained largely unknown.
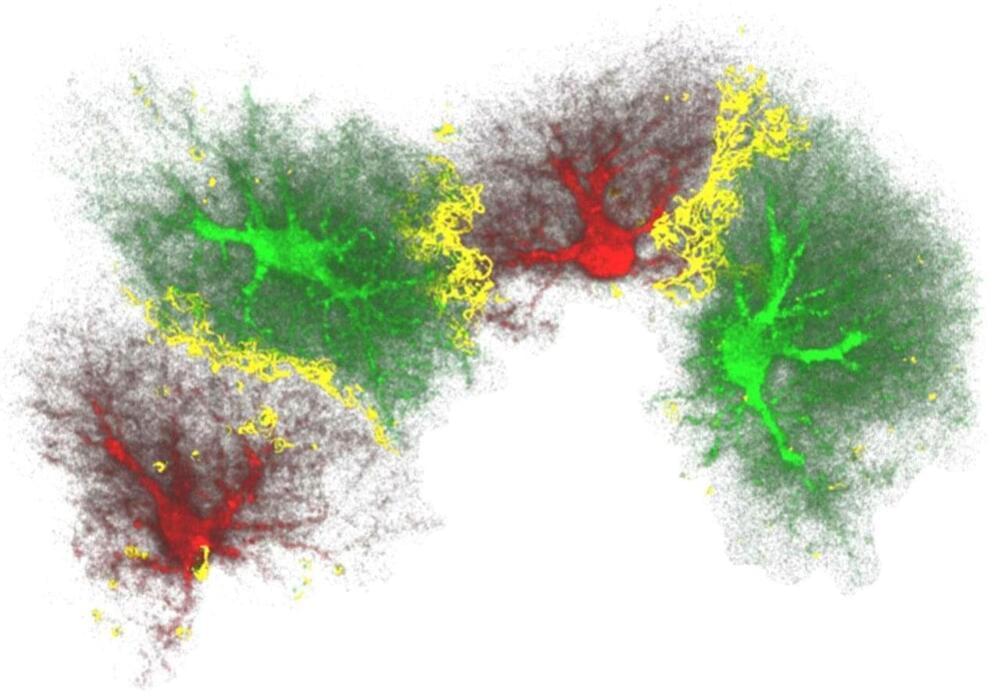
New insight into the generation of new neurons in the adult brain
Researchers at Sahlgrenska Academy at the University of Gothenburg, Sweden, in collaboration with research groups in Finland, Canada and Slovenia, have discovered a novel and unexpected function of nestin, the best-known marker of neural stem cells.
In the developing brain, the three main cell types, neurons, astrocytes and oligodendrocytes, are generated from neural stem cells. In some parts of the brain such as the hippocampus, the brain region involved in learning and memory, new neurons are being added to the existing neuronal circuitry even in adulthood, when severe restriction of neuronal differentiation occurs.
Using mice deficient in nestin, a protein that is a component of the part of the cytoskeleton known as intermediate filaments or nanofilaments, the research team led by Prof. Milos Pekny showed that nestin produced in astrocytes has an important role in inhibiting neuronal differentiation. They linked this regulatory function of nestin to the Notch signaling from astrocytes to neighboring neural stem cells. Thus, surprisingly, nestin does not control the generation of neurons by acting within neural stem cells, but indirectly by regulating the neurogenesis-inhibitory Notch signals that neural stem cells receive from astrocytes, important constituents of the neurogenic niche.
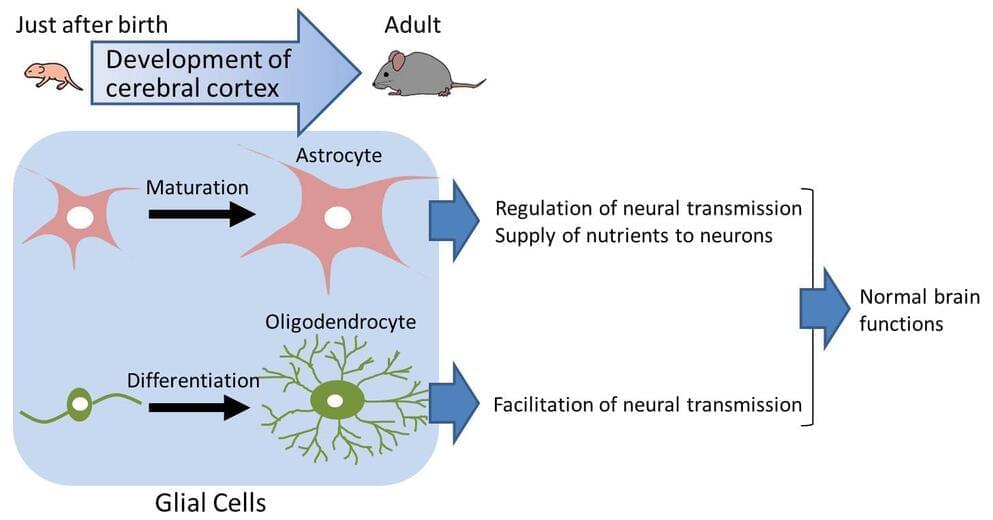
CD38 gene is identified to be important in postnatal development of the cerebral cortex
The brain consists of neurons and glial cells. The developmental abnormality of glial cells causes various diseases and aberrant cerebral cortex development. CD38 gene knockout is shown to cause aberrant development of glial cells, especially astrocytes and oligodendrocytes. The CD38 gene is known to be involved in cerebral cortex development. The present study suggests the importance of glial cells for cerebral cortex development.
It is essential for brain development that both neurons and glial cells develop in a normal manner not only during fetal but also postnatal stages. In the postnatal brain, neurons extend long protrusions (axons and dendrites) to form complex networks for information exchange. On the other hand, glial cells are thought to support network formation of neurons, to regulate transmission of information, and to help survival of neurons. It is known that more than 50 percent of total cells in the brain are glial cells, three times more than neurons in number. It is also known that in the human brain has far more glial cells than the brains of rodents or primates. This indicates that for the higher functions of the brain, glial cells are of particular importance.
In the past, research on developmental disorders of the brain focused on neurons. Recently, however, research has focused on the abnormality of glial cells. There remain a number of unsolved problems concerning the mechanism of glial cell development in the postnatal brain and the relationship of glial cell abnormalities and developmental disorders of the brain.
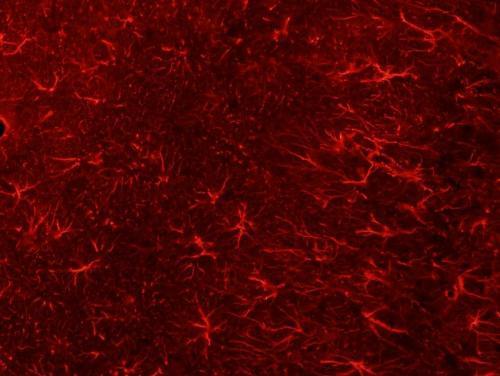
Adding human glial cells to mice brains found to improve memory and cognition
(Medical Xpress)—A team of researchers working at the University of Rochester in New York, has found that injecting glial cells into a mouse brain caused an improvement in both memory and cognition in the mouse. In their paper published in The Journal of Neuroscience, the team explains how they injected the test mice and then tested them afterwards to see what impact it had on their abilities.
Injecting human brain cells into mice brains appears to be the stuff of horror films, but in this case, it wasn’t really what it might have seemed. Glial cells are precursors to other cells—in this case, they develop into astrocytes, which are technically, brain cells. But, the important distinction here is that they are not neurons, which means they are not involved in thinking—instead they are involved in memory retention and help with housekeeping tasks.
Last year, the team injected mature glial cells into mice brains and reported improvements in ability by the mice—this time they went further, injecting progenitor precursor glial cells, which allows for development of more astrocytes. The team injected just 300,000 of the cells (from donated human embryos) and found just 12 months later that they had multiplied to grow to 12 million, completely displacing the original mouse astrocytes. It appeared, the team reported, that the cell growth only stopped when it reached the physical confines of the skull. They also note that it was interesting that the glial could thrive in such an environment considering that astrocytess in people are 10 to 20 times as big as those in mice and they carry 100 times as many tendrils. Testing the mice showed that their memory was far superior to normal mice and they had improved cognition as well.
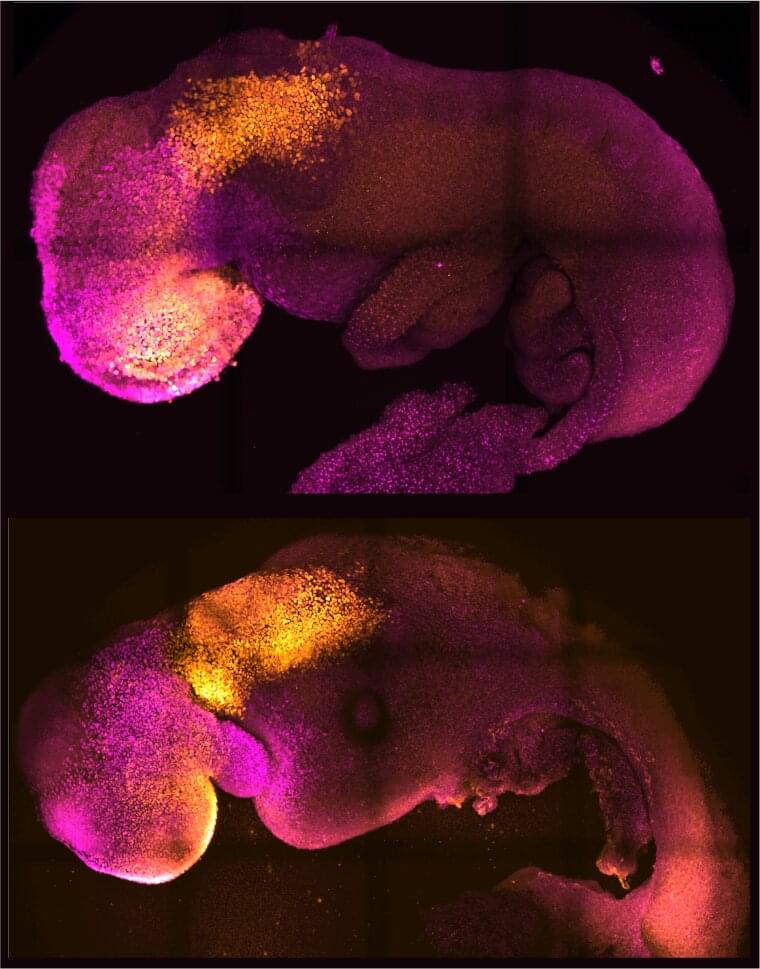
‘Synthetic’ mouse embryo with brain and beating heart grown from stem cells
Researchers from the University of Cambridge and Caltech have created model mouse embryos from stem cells—the body’s master cells, which can develop into almost any cell type in the body—that have beating hearts, as well as the foundations for a brain and all of the other organs in the mouse body.
The results are the culmination of more than a decade of research, and they could help researchers understand why some embryos fail while others go on to develop into a fetus as part of a healthy pregnancy. Additionally, the results could be used to guide repair and development of synthetic human organs for transplantation.
The research was conducted in the laboratory of Magdalena Zernicka-Goetz, Bren Professor of Biology and Biological Engineering at Caltech. Zernicka-Goetz is also a professor of mammalian development and stem cell biology in Cambridge’s Department of Physiology, Development and Neuroscience. A paper describing the breakthrough appears in the journal Nature on August 25.