Miniaturized electrode caps are fabricated and used for 3D electrical recording from brain organoids.

To capture a broader understanding of memory encoding, we expanded our experiments to include two other stimulus types: colors and face pictures (see Materials and Methods). Both monkeys demonstrated high accuracy in memorizing grating orientations in the “orientation DMTS” task, colors in the “color DMTS” task, and face pictures in the “face DMTS” task [DP: ~94% and DQ: ~87% versus 50%, all P < 0.01 (one-sample t test)] (fig. S1), indicating that they had been well trained.
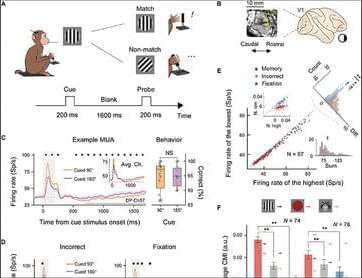
We implanted a Utah array in each monkey’s V1 area (see Materials and Methods; Fig. 1B) and presented the stimuli onto the receptive field (RF) centers of the recorded neurons (fig. S2, A and D). This enabled simultaneous monitoring of neuronal activity in our experiments. Our analyses focused primarily on neuronal activity before probe stimulus onset.
Representative neuronal responses for two of the VWM content conditions in the orientation DMTS task at a selected electrode are shown in Fig. 1C. During the stimulus period (0 to 200 ms after cue onset), neurons displayed distinct firing patterns between the two content conditions (90° or 180° orientation). An off-response emerged following the cue offset, and activity gradually diminished. During the delay period, defined as 700 to 1,700 ms after cue onset (the thick gray line in Fig. 1C), neurons also exhibited a significant difference in firing rate between the two content conditions (N = 1,810 trials for 90°; N = 1,865 trials for 180°; all marked positions P < 0.01) without any behavioral performance bias (N = 16 sessions, P = 0.94; right panel in Fig. 1C). The difference in response between these two content conditions during the delay period at the same electrode was less prominent in incorrect-response trials and in the fixation task (Fig. 1D).
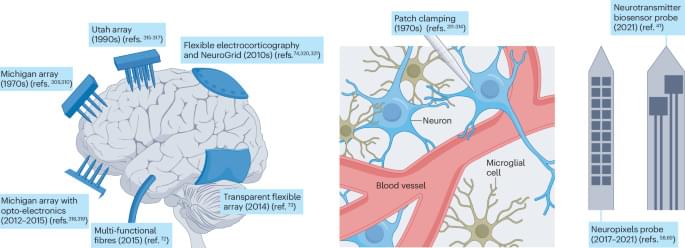
Brain-on-a-chip models, mimicking brain physiology, hold promise for developing treatments for neurological disorders. This Review discusses the engineering challenges and opportunities for these devices, including the integration of 3D cell cultures and electrodes and scaffold engineering strategies.

One of the ambitions of computational neuroscience is that we will continue to make improvements in the field of artificial intelligence that will be informed by advances in our understanding of how the brains of various species evolved to process information. To that end, here the authors propose an expanded version of the Turing test that involves embodied sensorimotor interactions with the world as a new framework for accelerating progress in artificial intelligence.

Nearly all the neural networks that power modern artificial intelligence tools such as ChatGPT are based on a 1960s-era computational model of a living neuron. A new model developed at the Flatiron Institute’s Center for Computational Neuroscience (CCN) suggests that this decades-old approximation doesn’t capture all the computational abilities that real neurons possess and that this older model is potentially holding back AI development.

A UK teenager with severe epilepsy has become the first person in the world to be fitted with a brain implant aimed at bringing seizures under control.
Oran Knowlson’s neurostimulator sits under the skull and sends electrical signals deep into the brain, reducing his daytime seizures by 80%.
His…
His mother, Justine, said that her son had been happier, chattier and had a much better quality of life since receiving the device. “The future looks hopeful, which I wouldn’t have dreamed of saying six months ago,” she said.
Martin Tisdall, a consultant paediatric neurosurgeon who led the surgical team at Great Ormond Street hospital (Gosh) in London, said: “For Oran and his family, epilepsy completely changed their lives and so to see him riding a horse and getting his independence back is absolutely astounding. We couldn’t be happier to be part of their journey.”
Oran, who is 13 and lives in Somerset, had the surgery in October as part of a trial at Gosh in partnership with University College London, King’s College hospital and the University of Oxford. Oran has Lennox-Gastaut syndrome, external, a treatment-resistant form of epilepsy which he developed at the age of three.
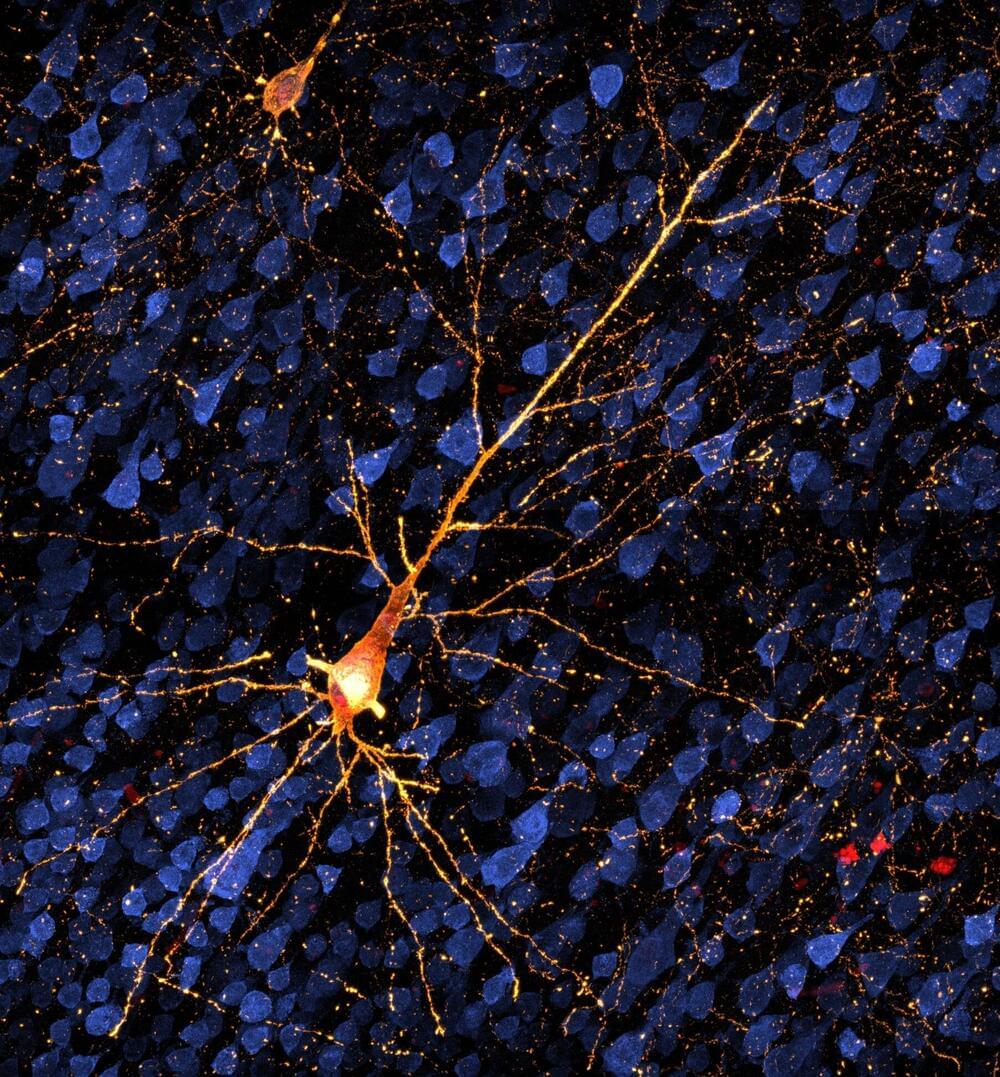
Research in nonhuman primates is opening the possibility of testing treatments for the early stages of Alzheimer’s and similar diseases, before extensive brain cell death and dementia set in. A study published in Alzheimer’s & Dementia shows up to a six-month window in which disease progress could be tracked and treatments tested in rhesus macaques.
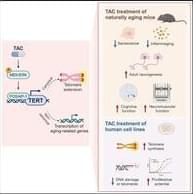
This study identifies a small molecule (TAC) that restores physiological levels of TERT throughout aged tissues, resulting in the rejuvenation of multiple tissues. Specifically, TAC administration in very aged mice alleviates multiple aging hallmarks such as cellular senescence and systemic inflammation, promotes new neuron formation with improved cognitive ability, enhances neuromuscular function, and is well tolerated with no evidence of toxicity.