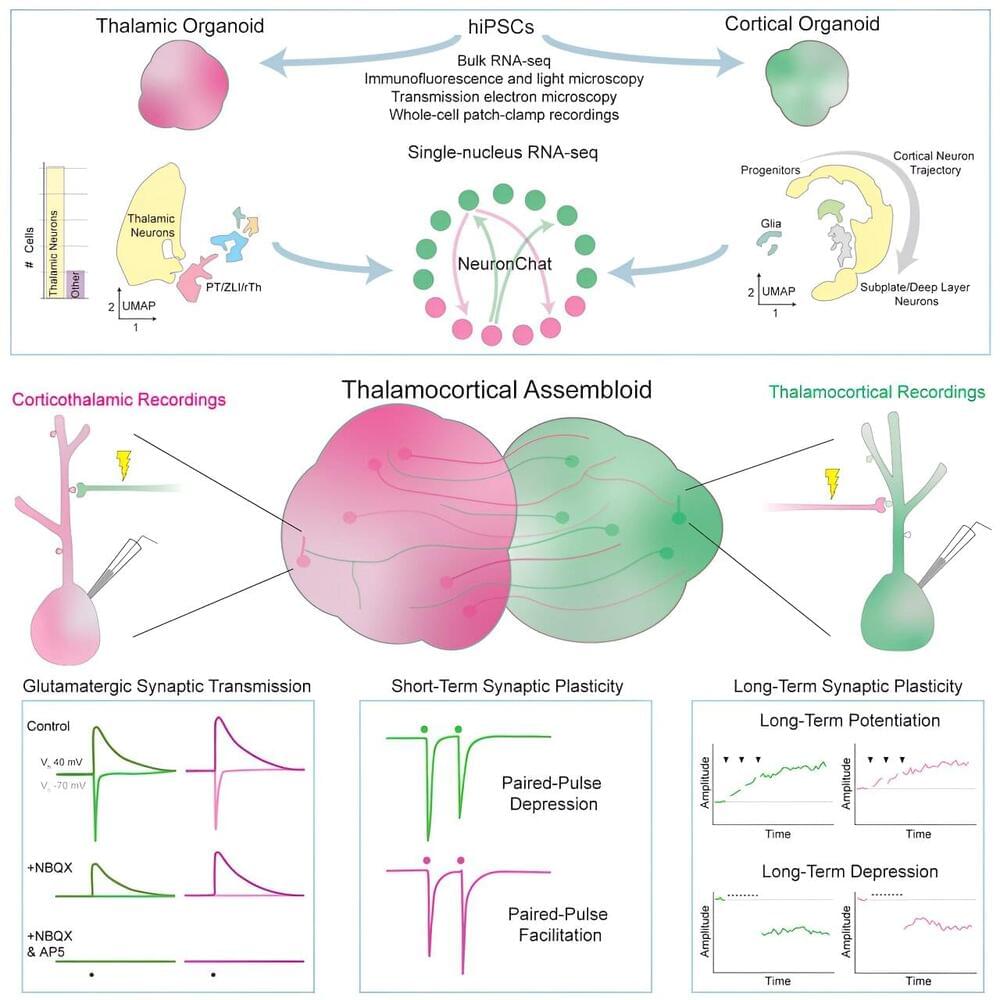
The ability to study human neurological systems depends on having viable, accurate models of brain function. St. Jude researchers have now created a model for such research by combining thalamic cells and cortical cells derived from human induced pluripotent stem cells.
The thalamocortical system mediates multiple sensory and cognitive processes, such as perception, learning and memory. The researchers developed a model of a primitive human thalamocortical system by maintaining thalamic and cortical cell masses known as organoids in close proximity in a culture dish.
In this model, the neurons in both organoids develop and grow long-ranging processes (axons) that extend to the opposite organoid and form functional connections (synapses). The researchers determined that when these synapses are stimulated, they undergo long-term strengthening and weakening of their electrical signals, which is the hallmark of synaptic plasticity, a process that underlies certain forms of learning and memory.