The social network company hopes neuroscience will give it an advantage in the AI race.


Researchers recently discovered that eight different psychiatric conditions share a common genetic basis.
A new study has now honed in on some of those shared genetic variants to understand their properties. They found many are active for longer during brain development and potentially impact multiple stages, suggesting they could be new targets to treat multiple conditions.
“The proteins produced by these genes are also highly connected to other proteins,” explains University of North Carolina geneticist Hyejung Won. “Changes to these proteins in particular could ripple through the network, potentially causing widespread effects on the brain.”

A recent study from the University of California San Diego School of Medicine has provided fresh insight into the potential benefits of time-restricted feeding in managing these circadian disruptions.
This approach, which involves eating within a specific daily window, could offer a novel way to address Alzheimer’s symptoms and possibly alter the course of the disease itself. The findings challenge traditional perspectives on the disorder, shifting attention to the importance of daily eating habits.
The circadian rhythm functions as the body’s internal biological clock, regulating numerous physiological processes, including the sleep-wake cycle. Disruptions to this rhythm are particularly common among Alzheimer’s patients, with recent estimates suggesting that up to 80% experience these disturbances. These disruptions not only interfere with sleep but also contribute to increased cognitive impairment, particularly during nighttime hours.
Summary: Scientists have discovered that neural stem cells (NSCs) receive constant feedback from their daughter cells, influencing whether they remain dormant or activate to form new neurons and glia. This parent-child relationship helps regulate brain regeneration and repair.
The study also reveals that calcium signaling plays a key role in how NSCs decode multiple signals from their environment. If NSCs produce only a few daughter cells, they activate; if they produce many, they stay dormant.
These findings challenge previous assumptions that NSCs function independently and open new avenues for treating neurodevelopmental disorders. Future research will explore how these processes change in aging and disease.
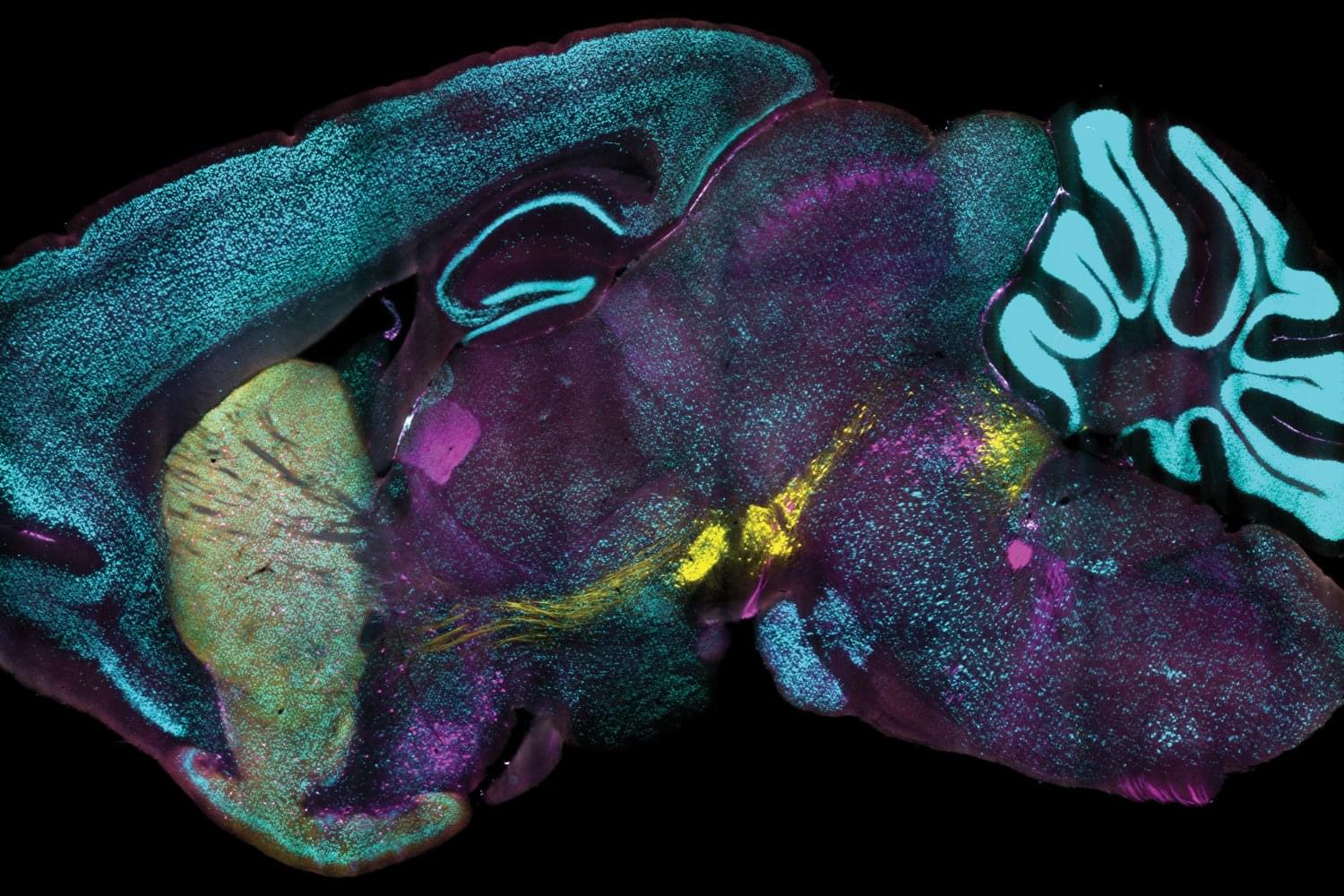
A new technology developed at MIT enables scientists to label proteins across millions of individual cells in fully intact 3D tissues with unprecedented speed, uniformity, and versatility. Using the technology, the team was able to richly label large tissue samples in a single day. In their new study in Nature Biotechnology, they also demonstrate that the ability to label proteins with antibodies at the single-cell level across large tissue samples can reveal insights left hidden by other widely used labeling methods.
Profiling the proteins that cells are making is a staple of studies in biology, neuroscience, and related fields because the proteins a cell is expressing at a given moment can reflect the functions the cell is trying to perform or its response to its circumstances, such as disease or treatment. As much as microscopy and labeling technologies have advanced, enabling innumerable discoveries, scientists have still lacked a reliable and practical way of tracking protein expression at the level of millions of densely packed individual cells in whole, 3D intact tissues. Often confined to thin tissue sections under slides, scientists therefore haven’t had tools to thoroughly appreciate cellular protein expression in the whole, connected systems in which it occurs.
“Conventionally, investigating the molecules within cells requires dissociating tissue into single cells or slicing it into thin sections, as light and chemicals required for analysis cannot penetrate deep into tissues. Our lab developed technologies such as CLARITY and SHIELD, which enable investigation of whole organs by rendering them transparent, but we now needed a way to chemically label whole organs to gain useful scientific insights,” says study senior author Kwanghun Chung, associate professor in The Picower Institute for Learning and Memory, the departments of Chemical Engineering and Brain and Cognitive Sciences, and the Institute for Medical Engineering and Science at MIT. “If cells within a tissue are not uniformly processed, they cannot be quantitatively compared. In conventional protein labeling, it can take weeks for these molecules to diffuse into intact organs, making uniform chemical processing of organ-scale tissues virtually impossible and extremely slow.”

A new Yale study provides a fuller picture of the genetic changes that shaped the evolution of the human brain, and how the process differed from the evolution of chimpanzees.
For the study, published Jan. 30 in the journal Cell, researchers focused on a class of genetic switches known as Human Accelerated Regions (HARs), which regulate when, where, and at what level genes are expressed during evolution.
While past research theorized that HARs may act by controlling different genes in humans compared to chimpanzees, our closest primate relative, the new findings show that HARs fine-tune the expression of genes that are already shared between humans and chimpanzees, influencing how neurons are born, develop, and communicate with each other.

Uniquely human features of neocortical development and maturation are not only intriguing for their implications in human-specific cognitive abilities, but they are also vulnerable to dysregulation which could cause or contribute to distinctly human brain disorder pathophysiology. The human cerebral cortex is essential for both cognition and emotional processing and dysregulation of these processes of the cortex are associated with a wide range of brain disorders including schizophrenia (SZ), autism spectrum disorder (ASD), Parkinson’s disease (PD), and Alzheimer’s disease (AD) (Berman and Weinberger, 1991; Rubenstein, 2011; Xu et al., 2019). Much remains to be learned about the mechanisms governing cortical expansion and responses to pathogenesis between human and non-human primates (NHPs) (Otani et al., 2016). Understanding these differences could shed light on the underlying mechanisms responsible for human-specific brain disorders and lead to the identification of key targets for the development of effective therapies.
Subtle differences observed by comparing human neurodevelopment to that of our closest evolutionary relatives could reveal underlying mechanisms, including genomic or transcriptional differences, contributing to varied phenotypes (Pollen et al., 2019). Human-specific responses to pathogenesis might be elucidated in a similar manner; by comparing brain pathophysiology of humans to our non-human primate counterparts (Hof et al., 2004). Although rodent models have taught us much about basic mammalian brain development and disorders (Fernando and Robbins, 2011), comparing governing processes and responses to species more closely related to humans can reduce the number of variables allowing for the identification of specific mechanisms responsible for observed deviations. Studies analyzing induced pluripotent stem cells (iPSCs) derived from humans, chimpanzees, and bonobos (Pan paniscus) show large sets of differentially expressed genes between human and NHP iPSCs. Perhaps the most compelling differentially expressed genes are those related to increased long interspersed element-1 (LINE-1) mobility in chimpanzees and bonobos, which could have implications on the rates of genetic divergence among species, and alternative mechanisms of pluripotency maintenance in chimpanzees (Marchetto et al., 2013; Gallego Romero et al., 2015). Furthermore, when human and NHP iPSCs were differentiated to neurons, they displayed distinctive migratory patterns at the neural progenitor cell (NPC) stage followed by contrasting morphology and timing of maturation in neurons (Marchetto et al., 2019). Despite the ability of two-dimensional (2D) PSC-derived neural cultures to demonstrate basic organization and transcriptomic changes of early brain development (Yan et al., 2013), while retaining the genetic background of the somatic cells from which they are reprogrammed, they lack the ability to develop complex cytoarchitecture, recapitulate advanced spatiotemporal transcriptomics, and brain region interconnectivity (including migration and axon guidance) of ensuing primate brain development (Soldner and Jaenisch, 2019). Intricate cellular heterogeneity, complex architecture, and interconnectivity of neurodevelopment, in addition to pathogenic responses, could be observed by comparing human and NHP brain tissues; however, ethical concerns and the inaccessibility of pre-and postnatal primate brain tissues limits the feasibility of such studies.
While brain organoids might be a long way from forming or sharing thoughts with us, they could still teach us much about ourselves. Brain organoids are three-dimensional (3D), PSC-derived structures that display complex radial organization of expanding neuroepithelium following embedding in an extracellular matrix like Matrigel and can recapitulate some subsequent processes of neurodevelopment including neurogenesis, gliogenesis, synaptogenesis, heterogenous cytoarchitecture, cell and axon migration, myelination of axons, and spontaneously-active neuronal networks (Lancaster et al., 2013; Bagley et al., 2017; Birey et al., 2017; Quadrato et al., 2017; Xiang et al., 2017; Marton et al., 2019; Shaker et al., 2021). It is likely that all these features of neurodevelopment are governed by some degree of specifies-specific dynamics. Brain organoids can be generated from human and NHP PSCs and, since some pathways regulating neural induction and brain region specification are well conserved in primates, both unguided cerebral organoids and guided brain region specific organoids can be generated (Mora-Bermúdez et al., 2016; Field et al., 2019; Kanton et al., 2019). Additional protocols have been established for the derivation of brain region specific organoids from human PSCs (hPSCs), including dorsal forebrain, ventral forebrain, midbrain, thalamus, basal ganglia, cerebellum, and telencephalic organoids (Muguruma et al., 2015; Sakaguchi et al., 2015; Jo et al., 2016; Bagley et al., 2017; Birey et al., 2017; Watanabe et al., 2017; Xiang et al., 2017, 2019; Qian et al., 2018). With some modifications, these methods could prove to be successful in establishing brain region-specific organoids from a variety of NHP PSC lines allowing for the reproducible comparison of homogeneous, human-specific neurodevelopment and brain disorder pathophysiology in brain regions beyond the cortex.
