Equips yourself with the latest thinking in neuroscience, offering you a groundbreaking approach to personal effectiveness, creativity, social connections, decision making, and organizational performance.



Most neuroscience research carried out up to date has primarily focused on neurons, the most renowned type of cell in the human brain. As a result, the unique functions of other brain cell types are less understood and have often been entirely overlooked.
Researchers at Instituto Cajal (CSIC), the Autonomous University of Madrid and Institute de Salud Carlos III recently carried out a study aimed at better understanding the contributions of astrocytes, a class of star-shaped glial cells found in the brain and spinal cord, to key mental functions. Their findings, published in Nature Neuroscience, unveiled the existence of astrocytic ensembles, specialized astrocyte subsets that appear to be active during reward-driven behaviors.
“It is known that astrocytes are a heterogeneous cell type in their molecular and gene expression signatures, morphology and origin,” Marta Navarrete, senior author of the paper, told Medical Xpress.
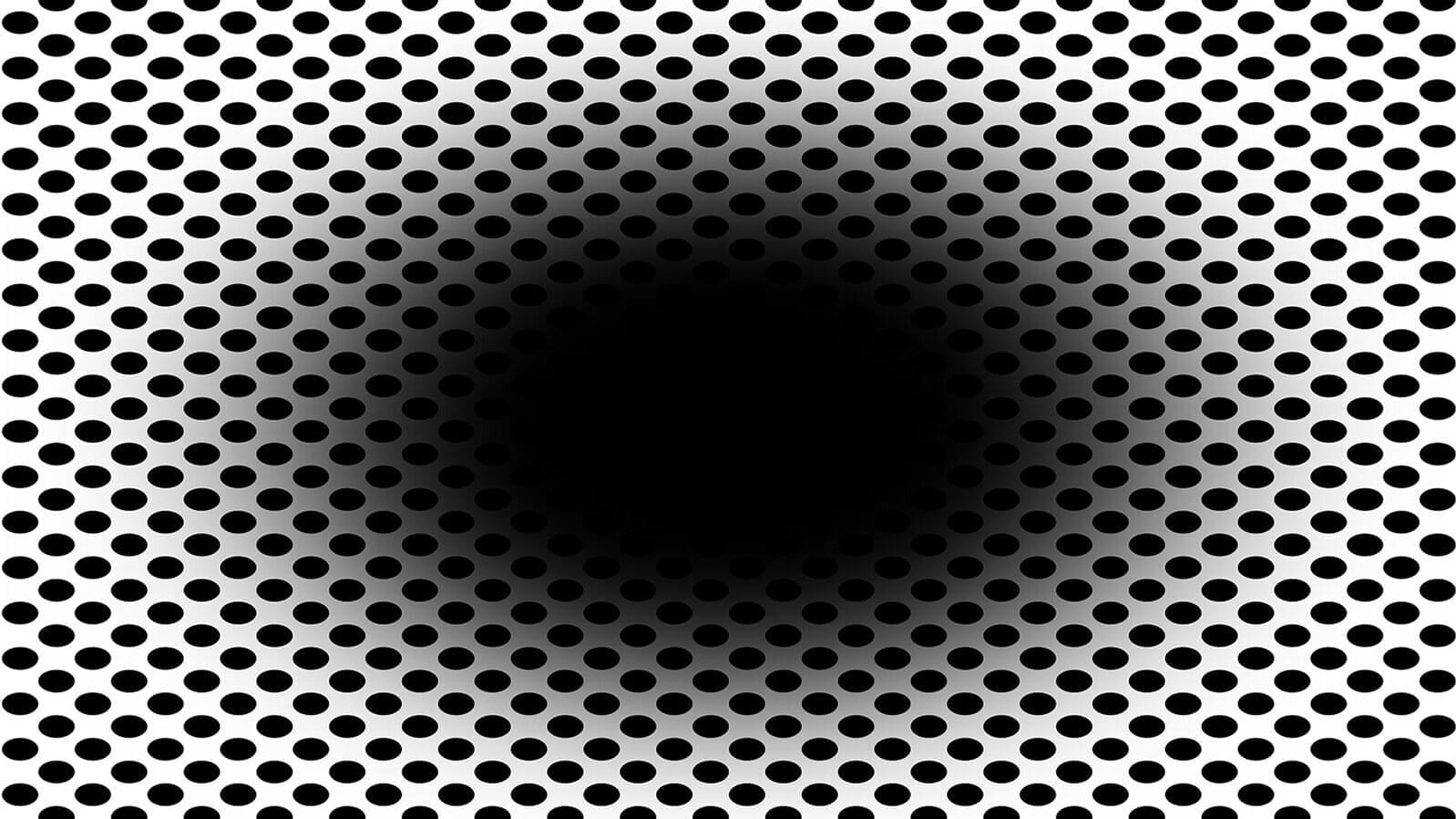
Our brain and eyes can play tricks on us—not least when it comes to the expanding hole illusion. A new computational model developed by Flinders University experts helps to explain how cells in the human retina make us “see” the dark central region of a black hole graphic expand outwards.
In a new article posted to the arXiv preprint server, the Flinders University experts highlight the role of the eye’s retinal ganglion cells in processing contrast and motion perception—and how messages from the cerebral cortex then give the beholder an impression of a moving or “expanding hole.”
“Visual illusions provide valuable insights into the mechanisms of human vision, revealing how the brain interprets complex stimuli,” says Dr. Nasim Nematzadeh, from the College of Science and Engineering at Flinders University.
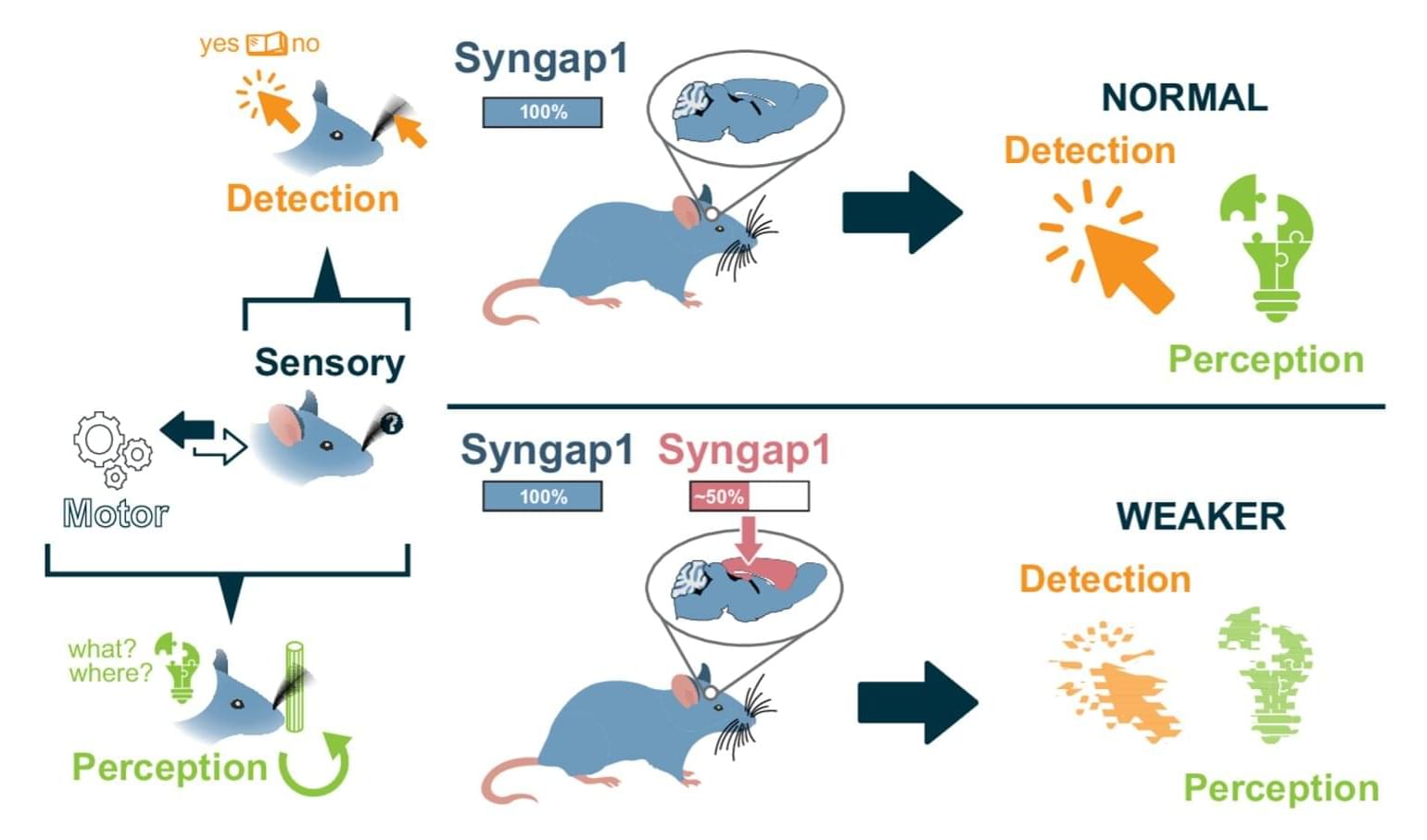
The SYNGAP1 gene, which supports the production of a protein called SynGAP (Synaptic Ras GTPase-Activating Protein), is known to play a key role in supporting the development of synapses and neural circuits (i.e., connections between neurons). Mutations in this gene have been linked to various learning disabilities, including intellectual disabilities, speech and language delays, autism spectrum disorder (ASD), and epilepsy.
Researchers at the Herbert Wertheim UF Scripps Institute for Biomedical Innovation & Technology recently carried out a study aimed at better understanding the genetic mechanisms via which the SYNGAP1 gene contributes to healthy cognitive function. Their findings, published in Nature Communications, suggest that the autonomous expression of this gene in the cortical excitatory neurons of mice promotes the animals’ cognitive abilities via the assembly of long-range neural circuits integrating sensory and motor information.
“Our paper builds on our ongoing research into how major risk genes for mental health disorders, including autism, regulate brain organization and function,” Gavin Rumbaugh, senior author of the paper, told Medical Xpress. “The field knows the major risk genes that directly contribute to cognitive and behavioral impairments that lead to diagnosable forms of autism and related neuropsychiatric disorders in humans.
In cognitive warfare truth becomes lies good becomes evil.
Provided to YouTube by Routenoteall the collapsing stars · sarochi · sarochi (ken)sarochi℗ SAROCHIReleased on: 2025–02-05Auto-generated by YouTube.
To see how cognitive maps form in the brain, researchers used a Janelia-designed, high-resolution microscope with a large field of view to image neural activity in thousands of neurons in the hippocampus of a mouse as it learned. Credit: Sun and Winnubst et al.
Our brains build maps of the environment that help us understand the world around us, allowing us to think, recall, and plan. These maps not only help us to, say, find our room on the correct floor of a hotel, but they also help us figure out if we’ve gotten off the elevator on the wrong floor.
Neuroscientists know a lot about the activity of neurons that make up these maps – like which cells fire when we’re in a particular location. But how the brain creates these maps as we learn remains a mystery.

Read “” by Sebastian Schepis on Medium.
Imagine a world where thoughts aren’t confined to the brain, but instantly shared across a vast network of neurons, transcending the limits of space and time. This isn’t science fiction, but a possibility hinted at by one of the most puzzling aspects of quantum physics: entanglement.
Quantum entanglement, famously dubbed spooky action at a distance by Einstein, describes a phenomenon where two or more particles become intrinsically linked. They share a quantum state, no matter how far apart they are. Change one entangled particle, and its partner instantly reacts, even across vast distances.
This property, which troubled Einstein, has been repeatedly confirmed through experiments, notably by physicist John Clauser and his colleagues, who received the 2022 Nobel Prize in Physics for their groundbreaking work on quantum entanglement.


Thanks to their excellent smelling ability, dogs have been used for hundreds of years to hunt down wild game and search for criminals. At airports, they help identify explosives and illicit drugs. In disaster situations, they can rescue survivors and find human remains.
But each dog can only be trained to detect one class of odor compounds, which limits the range of smells it’s able to detect. Training costs tens of thousands of dollars and takes several months. For Florida startup Canaery, the solution is merging canines with neurotechnology to allow them to detect everything from bombs and other contraband to human diseases and environmental toxins—no specialized training needed.
Using in vitro models to study human brain evolution and diseaseCSAR lecture by Dr. Madeline Lancaster, MRC Laboratory of Molecular BiologyThe human brain is…