Lecture by Jeffrey D. Schall, Ph.D.
Category: neuroscience – Page 287

Your Vision Can Predict Dementia 12 Years Before Diagnosis, Study Discovers
The eyes can reveal a lot about the health of our brain. Indeed, problems with the eyes can be one of the earliest signs of cognitive decline.
Our latest study shows that a loss of visual sensitivity can predict dementia 12 years before it is diagnosed.
Our research was based on 8,623 healthy people in Norfolk, England, who were followed up for many years. By the end of the study, 537 participants had developed dementia, so we could see what factors might have preceded this diagnosis.
Breaking the Memory Code: New Research Reveals What Makes Human Consciousness Unique
Surprising research from Spain has demonstrated the uniqueness of human consciousness, as a team of scientists say they have shown how the human brain stores memories differently than other species.
Neurons in a human brain record information separate from context, allowing humans to process more complex and abstract information relationships than other species. Dr. Rodrigo Quian Quiroga, group leader of the Neural Mechanisms of Perception and Memory Research Group at the Hospital del Mar Research Institute, led the groundbreaking research into human consciousness.
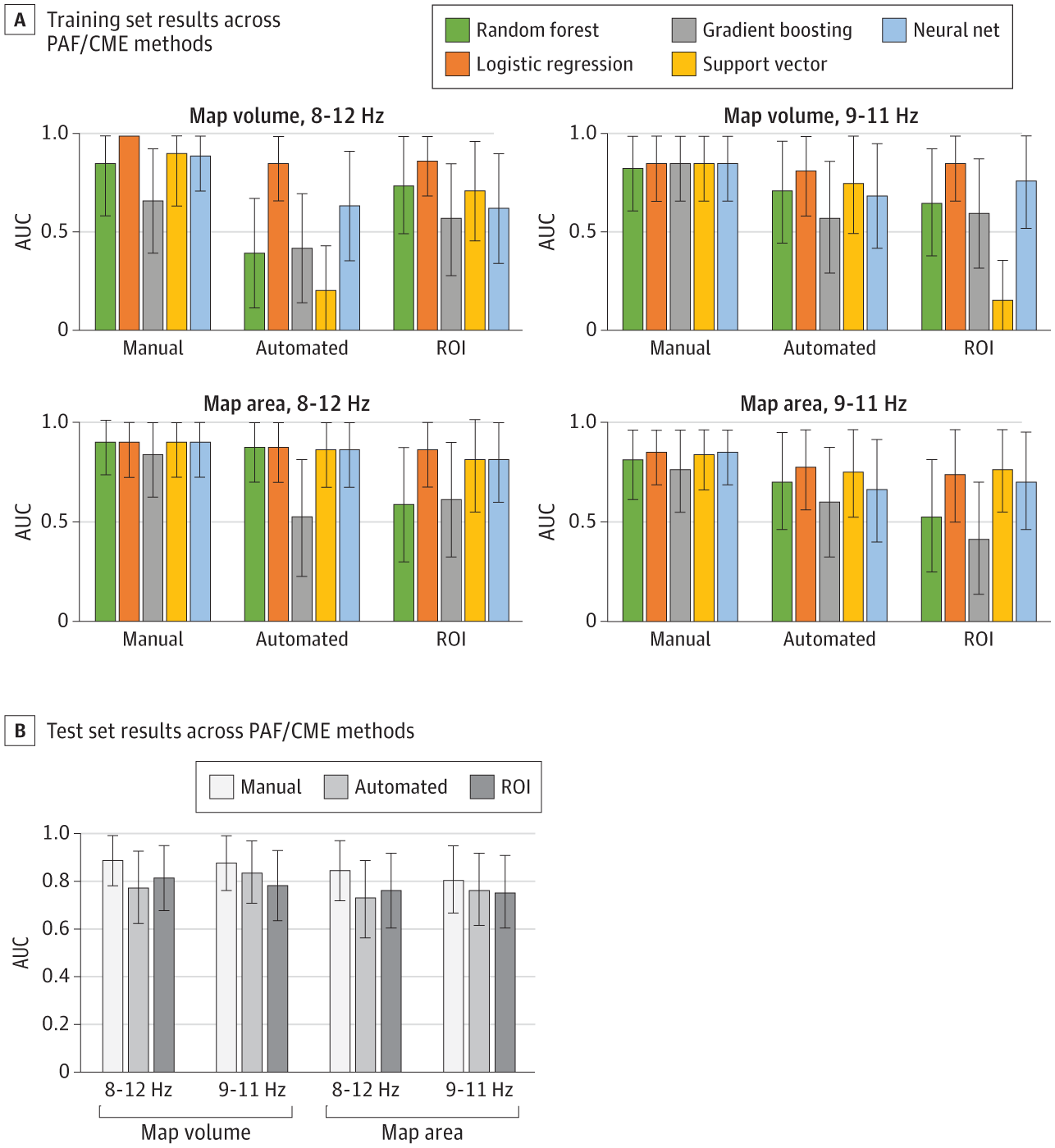
Predicting Individual Pain Sensitivity Using a Novel Cortical Biomarker Signature
A novel cortical biomarker can accurately distinguish high and low pain-sensitive individuals and may predict the transition from acute to chronic pain.
Importance Biomarkers would greatly assist decision-making in the diagnosis, prevention, and treatment of chronic pain.
Objective To undertake analytical validation of a sensorimotor cortical biomarker signature for pain consisting of 2 measures: sensorimotor peak alpha frequency (PAF) and corticomotor excitability (CME).
Design, Setting, and Participants This cohort study at a single center (Neuroscience Research Australia) recruited participants from November 2020 to October 2022 through notices placed online and at universities across Australia. Participants were healthy adults aged 18 to 44 years with no history of chronic pain or a neurological or psychiatric condition. Participants experienced a model of prolonged temporomandibular pain with outcomes collected over 30 days. Electroencephalography to assess PAF and transcranial magnetic stimulation (TMS) to assess CME were recorded on days 0, 2, and 5. Pain was assessed twice daily from days 1 through 30.
Dr. Courtney Millar — Marcus Inst. For Aging Research — Molecular Nutrition In Health & Well-Being
Molecular Nutrition In Health, Well-Being & Longevity — Dr. Courtney Millar, Ph.D. — Marcus Institute For Aging Research, Hebrew SeniorLife / Harvard Medical School
Dr. Courtney Millar, Ph.D. (https://www.marcusinstituteforaging.org/who-we-are/profiles/courtney-millar-phd) is an Assistant Scientist at the Hinda and Arthur Marcus Institute for Aging Research, Hebrew SeniorLife, and Instructor in Medicine, Harvard Medical School and Beth Israel Deaconess Medical Center.
Dr. Millar is a research scientist devoted to improving health and well-being of older adults through dietary interventions and her current research aims to test the ability of anti-inflammatory dietary strategies that promote both physical and emotional well-being in older adults.
Dr. Millar received her PhD in molecular nutrition at the University of Connecticut, where she developed a deep understanding of the relationship between dietary bioactive components and metabolic disease.
Dr. Millar’s post-doctoral fellowship focused on training related to conducting both nutritional epidemiological analyses and clinical interventions.
Study uncovers developmentally distinct neural architectures controlling avoidant behaviors
Over the course of their lives, humans and other animals typically learn to avoid situations and stimuli that are dangerous or are perceived as threatening. Past neuroscience studies have gathered evidence suggesting that the medial prefrontal cortex (mPFC), a brain region that plays a key role in learning and decision-making, also contributes to these learned threat responses.
Researchers at the University of California Los Angeles (UCLA) recently carried out a study aimed at better understanding how the gradual strengthening of neural connections during the brain’s development influences changes in the threat responses of mice.
Their findings, published in Nature Neuroscience, revealed that there are critical transitions during brain development that alter how the mPFC interacts with the nucleus accumbens (NAc) and basolateral amygdala (BLA), two brain regions involved in threat-based and emotional learning.
I tweet, therefore I am: a systematic review on social media use and disorders of the social brain
With rapid technological advances, social media has become an everyday form of human social interactions. For the first time in evolutionary history, people can now interact in virtual spaces where temporal, spatial, and embodied cues are decoupled from one another. What implications do these recent changes have for socio-cognitive phenotypes and mental disorders? We have conducted a systematic review on the relationships between social media use and mental disorders involving the social brain. The main findings indicate evidence of increased social media usage in individuals with psychotic spectrum phenotypes and especially among individuals with disorders characterized by alterations in the basic self, most notably narcissism, body dysmorphism, and eating disorders.

Two brain areas compete for control of memories, optogenetics study shows
Researchers at Ruhr University Bochum, Germany, have studied the impact of two brain areas on the nature of memory content. The team from the Department of Neurophysiology showed in rats how the so-called locus coeruleus and the ventral tegmental area permanently alter brain activity in the hippocampus region, which is crucial for the formation of memory.
The two areas compete with each other for influence to determine, for example, in what way emotionally charged and meaningful experiences are stored. Dr. Hardy Hagena and Professor Denise Manahan-Vaughan conducted the study using optogenetics. In the process, they genetically modified rats so that certain nerve cells could be activated or deactivated with light.
They published their findings in the journal Proceedings of the National Academy of Sciences.
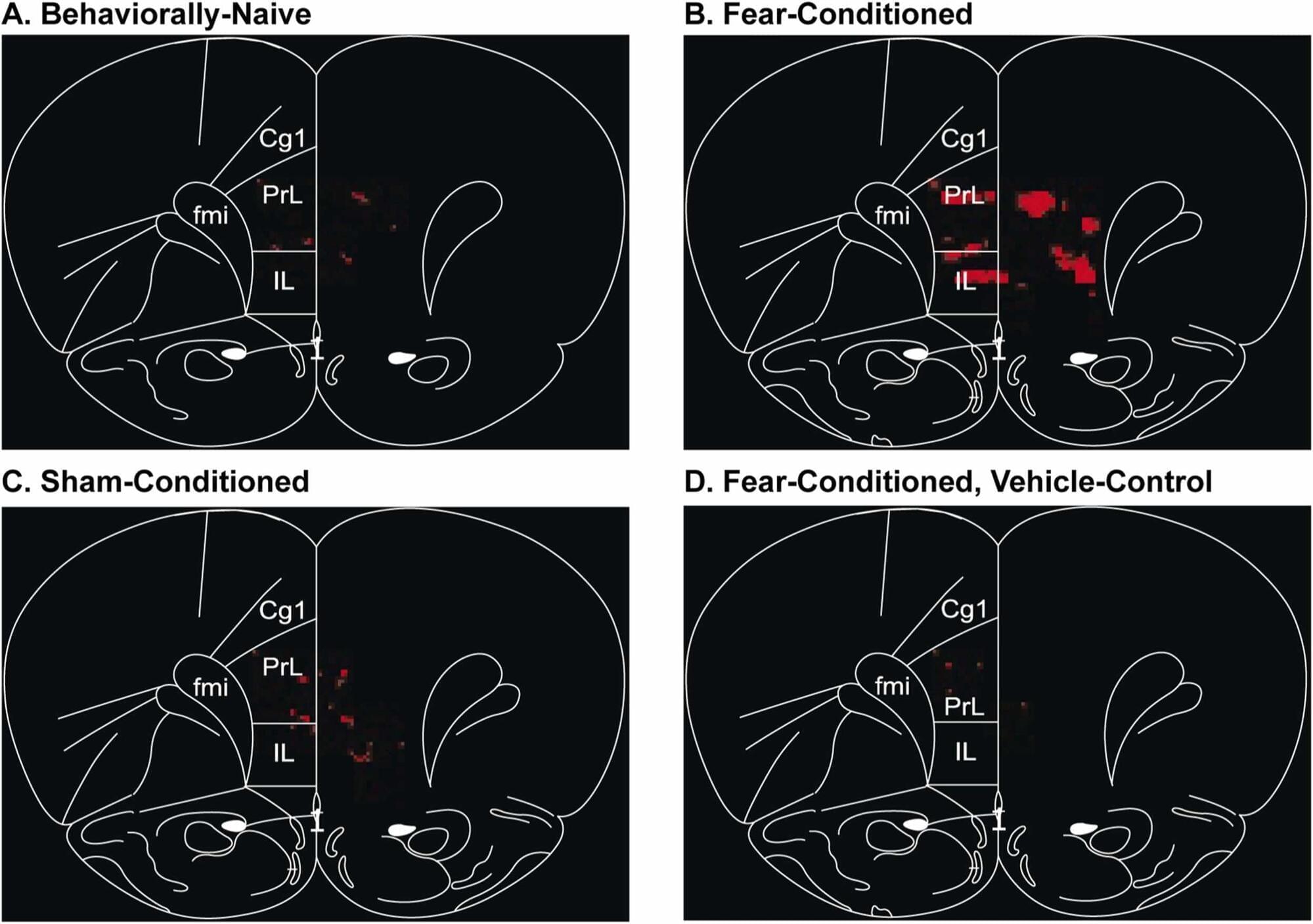
Photoacoustic imaging reveals new insights into how the brain learns new information
Wayne State University researchers are using photoacoustic imaging to observe brain activity and, in the process, discovering more about how it responds to different types of learning and experiences.
The team’s findings were recently published in the journal Photoacoustics.
The study, “Use of pattern recognition in photoacoustic imaging to identify neuronal ensembles in the prefrontal cortex of rats undergoing conditioned fear learning,” stemmed from a project by Wayne State University School of Medicine alumnus, James Matchynski, M.D., Ph.D., and was led by School of Medicine faculty members Shane Perrine, Ph.D., associate professor of psychiatry and behavioral neurosciences, and Alana Conti, Ph.D., professor of psychiatry and behavioral neurosciences and director of the Translational Neuroscience Program. The team collaborated with colleagues in the Department of Biomedical Engineering at the University of Illinois Chicago.