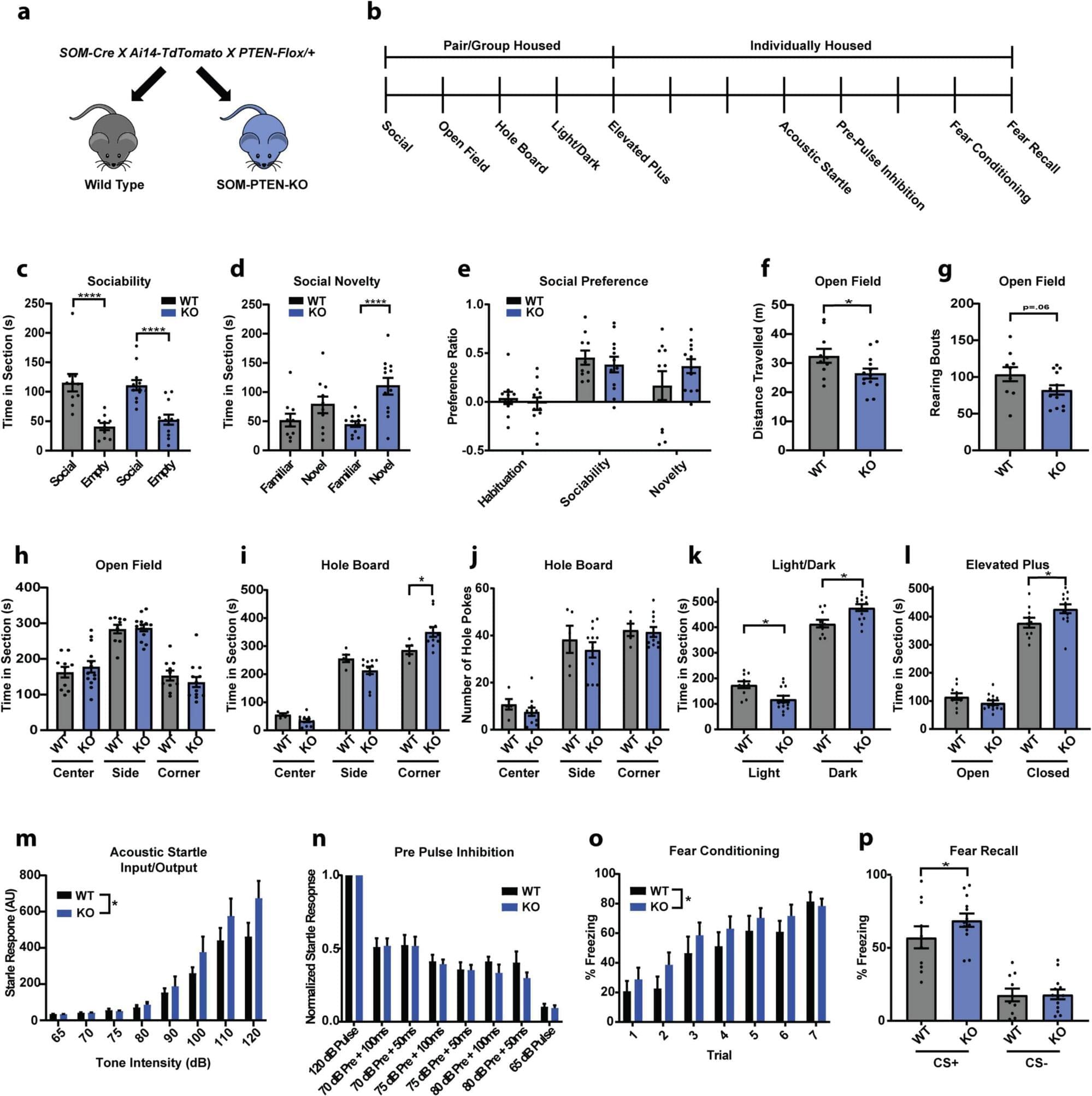
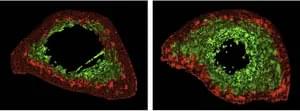
Researchers at the Max Planck Florida Institute for Neuroscience have discovered how loss of a gene strongly associated with autism and macrocephaly (large head size) rewires circuits and alters behavior.
Their findings, published in Frontiers in Cellular Neuroscience, reveal specific circuit changes in the amygdala resulting from PTEN loss in inhibitory neurons, providing new insights into the underlying circuit alterations that contribute to heightened fear and anxiety.
PTEN has emerged as one of the most significant autism risk genes. Variations in this gene are found in a significant proportion of people with autism who also exhibit brain overgrowth, making it a key player in understanding differences in brain function. To investigate the impact of PTEN misregulation, researchers have turned to animal models, where global reduction of PTEN results in altered sociability, repetitive behaviors, and increased anxiety that are often associated with ASD in humans.