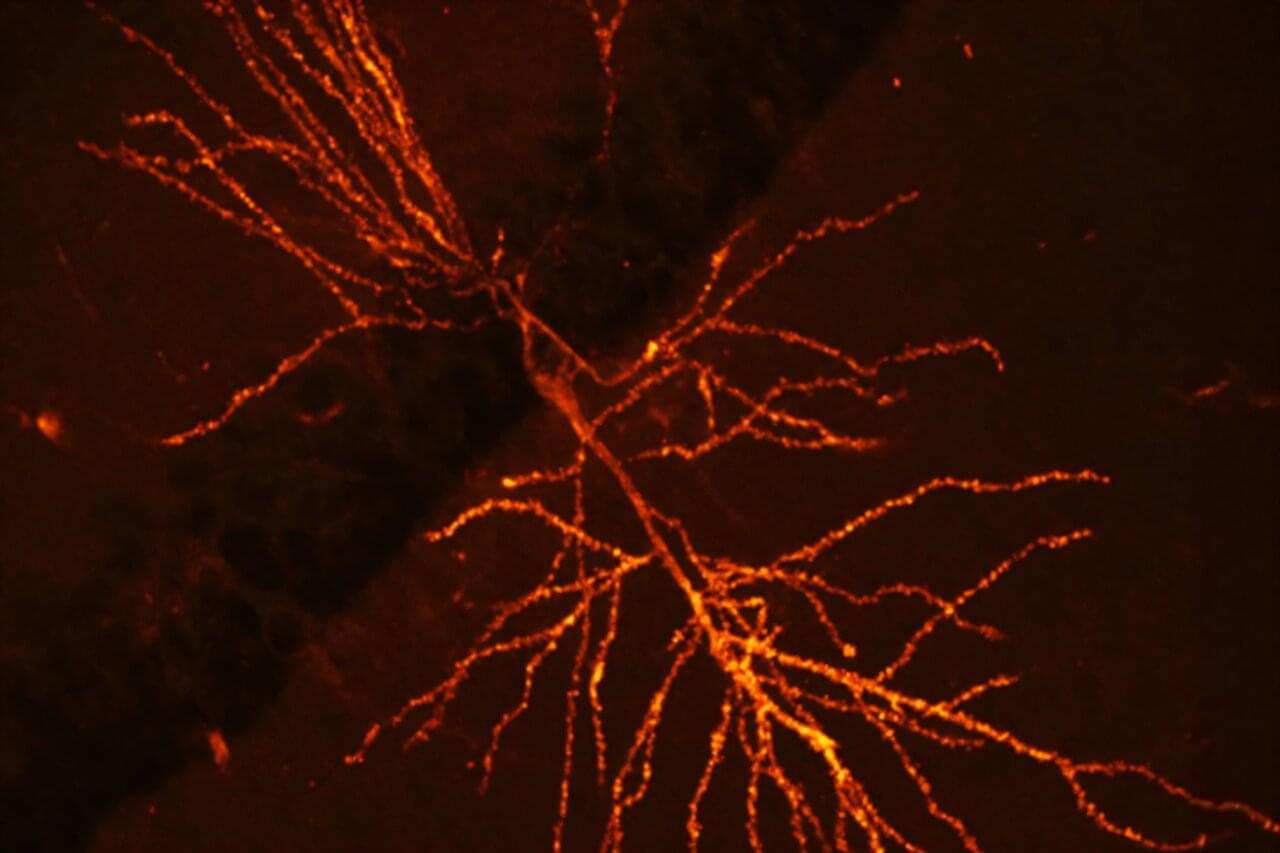
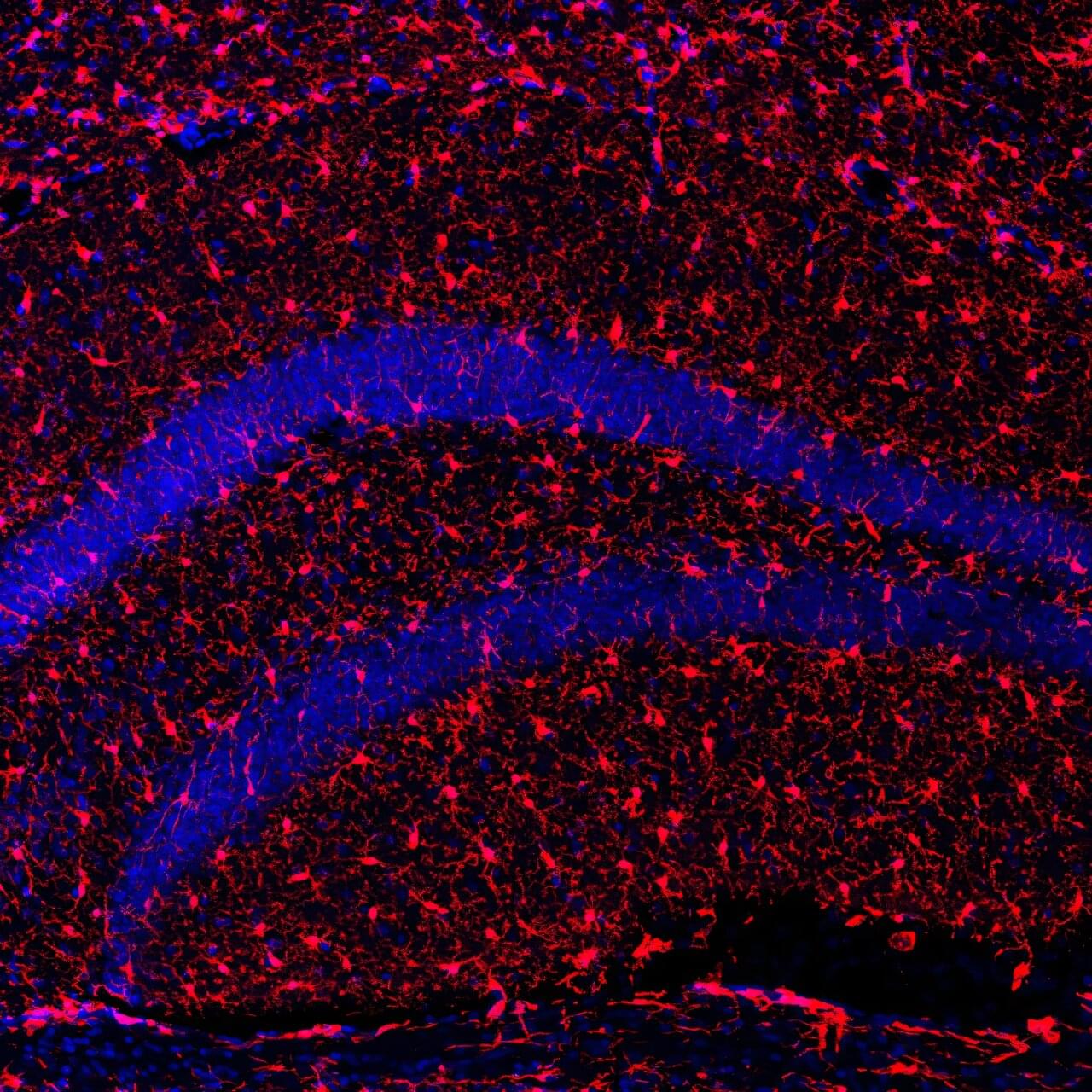
A team of Harvard researchers have unveiled a way to map the molecular underpinnings of how learning and memories are formed, a new technique expected to offer insights that may pave the way for new treatments for neurological disorders such as dementia.
“This technique provides a lens into the synaptic architecture of memory, something previously unattainable in such detail,” said Adam Cohen, professor of chemistry and chemical biology and of physics and senior co-author of the research paper, published in Nature Neuroscience.
Memory resides within a dense network of billions of neurons within the brain. We rely on synaptic plasticity—the strengthening and modulation of connections between these neurons—to facilitate learning and memory.