Using CryoEM, researchers capture a new video of dynein-Lis1 protein interaction, supporting future drug development for neurological disorders.

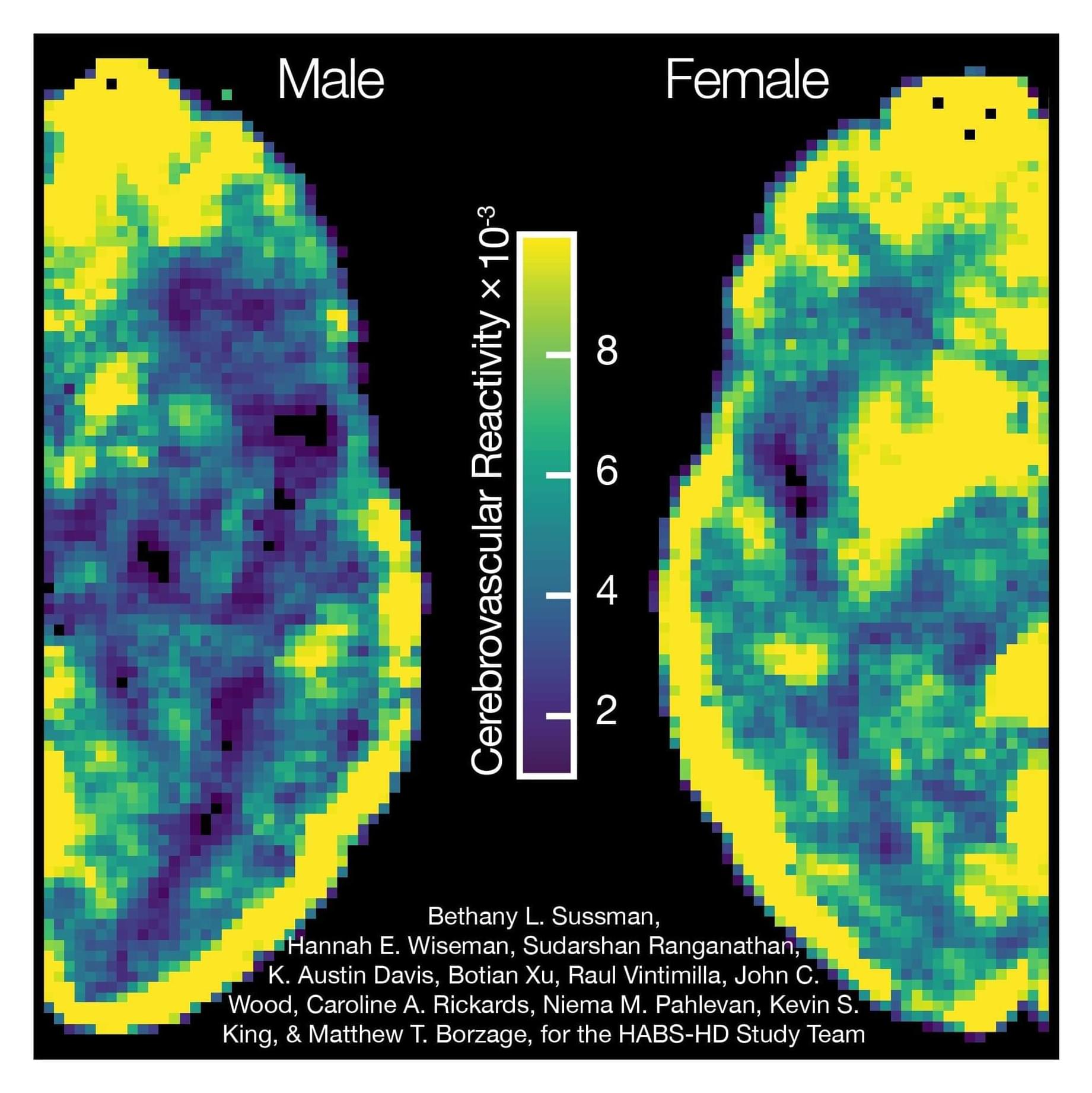
A research team led by the Borzage Laboratory at Children’s Hospital Los Angeles tested a new functional magnetic resonance imaging (fMRI) analysis method to measure cerebrovascular health in aging adults. What they found was unexpected and validated the usefulness of this method for measuring neurovascular aging in childhood diseases.
The researchers measured the cerebrovascular reactivity of the brains of 53 men and women between the ages of 51 to 83. Cerebrovascular reactivity is the ability of the blood vessels in the brain to dilate in response to a stimulus. The fMRI method they used—known as blood oxygen level dependent-cerebrovascular reactivity (BOLD-CVR)—measures the ability of the brain’s vessels to flexibly regulate blood flow in response to changes in carbon dioxide levels.
“How well the vessels react reveals a lot about your brain health,” says lead author Bethany Sussman, Ph.D., Research Scientist, Neonatology, at CHLA. “If a certain part of the brain can’t perform that function very well, that area is likely more susceptible to stroke.
Persons with Parkinson’s disease increasingly lose their mobility over time and are eventually unable to walk. Hope for these patients rests on deep brain stimulation, also known as a brain pacemaker.
In a current study, researchers at Ruhr University Bochum and Philipps-Universität Marburg, Germany, investigated whether and how stimulation of a certain region of the brain can have a positive impact on ambulatory ability and provide patients with a higher quality of life. To do this, the researchers used a technique in which the nerve cells are activated and deactivated via light. Their report is published in the journal Scientific Reports.

Learning and motivation are driven by internal and external rewards. Many of our day-to-day behaviours are guided by predicting, or anticipating, whether a given action will result in a positive (that is, rewarding) outcome. The study of how organisms learn from experience to correctly anticipate rewards has been a productive research field for well over a century, since Ivan Pavlov’s seminal psychological work. In his most famous experiment, dogs were trained to expect food some time after a buzzer sounded. These dogs began salivating as soon as they heard the sound, before the food had arrived, indicating they’d learned to predict the reward. In the original experiment, Pavlov estimated the dogs’ anticipation by measuring the volume of saliva they produced. But in recent decades, scientists have begun to decipher the inner workings of how the brain learns these expectations. Meanwhile, in close contact with this study of reward learning in animals, computer scientists have developed algorithms for reinforcement learning in artificial systems. These algorithms enable AI systems to learn complex strategies without external instruction, guided instead by reward predictions.
The contribution of our new work, published in Nature (PDF), is finding that a recent development in computer science – which yields significant improvements in performance on reinforcement learning problems – may provide a deep, parsimonious explanation for several previously unexplained features of reward learning in the brain, and opens up new avenues of research into the brain’s dopamine system, with potential implications for learning and motivation disorders.
Reinforcement learning is one of the oldest and most powerful ideas linking neuroscience and AI. In the late 1980s, computer science researchers were trying to develop algorithms that could learn how to perform complex behaviours on their own, using only rewards and punishments as a teaching signal. These rewards would serve to reinforce whatever behaviours led to their acquisition. To solve a given problem, it’s necessary to understand how current actions result in future rewards. For example, a student might learn by reinforcement that studying for an exam leads to better scores on tests. In order to predict the total future reward that will result from an action, it’s often necessary to reason many steps into the future.

Researchers have identified how variations in a gene called TRIO can influence brain functions and result in distinct neurodevelopmental diseases. The study, published in the journal eLife, could pave the way for future therapeutic developments.
TRIO encodes a diverse group of proteins that control the function and structure of the cytoskeleton—a cell’s internal scaffolding. Rare damaging variants in this gene have been identified in individuals with intellectual disability, autism spectrum disorder, schizophrenia, and related disorders. However, the mechanisms underlying the associations aren’t yet understood.
“It’s really extraordinary that different variants in this single gene can have such dramatically different effects on brain development and function,” says Anthony Koleske, Ph.D., Ensign Professor of Molecular Biophysics and Biochemistry at Yale School of Medicine (YSM) and the study’s senior author.

Slight variations in a person’s pulse rate could give clues about the likelihood of future cognitive decline, according to a new study, potentially giving us a valuable new test for cognitive problems that would be quick and easy to run.
This is something that researchers invest a lot of time in, because knowing when cognitive decline might start, and how it may progress, means better support and more clarity for those involved. Along the way, it also reveals new insights into how these conditions develop, and how they might be stopped for good.
In this study, an international team analyzed pulse rate data across a night of sleep from 503 individuals with an average age of 82. Cognitive tests were also carried out around that same time, as well as in at least one follow-up visit.
In recent years, scientists discovered something strange: When mice with Alzheimer’s disease inhale menthol, their cognitive abilities improve.
It seems the chemical compound can stop some of the damage done to the brain that’s usually associated with the disease.
In particular, researchers noticed a reduction in the interleukin-1-beta (IL-1β) protein, which helps to regulate the body’s inflammatory response – a response that can offer natural protection but one that leads to harm when it’s not controlled properly.
