Polarons are important nanoscale phenomena: a transient configuration between electrons and atoms (known as quasiparticles) that exist for only trillionths of a second.

Dotty graphene and doping: Whatever it takes for Russia’s record plasmonics to shine.
Physicists from MIPT and Vladimir State University, Russia, have achieved a nearly 90% efficiency converting light energy into surface waves on graphene. They relied on a laser-like energy conversion scheme and collective resonances. The paper came out in Laser & Photonics Reviews.
Manipulating light at the nanoscale is a task crucial for being able to create ultracompact devices for optical energy conversion and storage. To localize light on such a small scale, researchers convert optical radiation into so-called surface plasmon-polaritons. These SPPs are oscillations propagating along the interface between two materials with drastically different refractive indices — specifically, a metal and a dielectric or air. Depending on the materials chosen, the degree of surface wave localization varies. It is the strongest for light localized on a material only one atomic layer thick, because such 2D materials have high refractive indices.

Atomically thin, 2-D hexagonal boron nitride (h-BN) is a promising material whose protean ability to undergo phase transformations to strong, super lightweight, chemically stable, oxidation-resistant films makes them ideal for protective coatings, nanotechnology thermal applications, deep-UV light emitters, and much more.
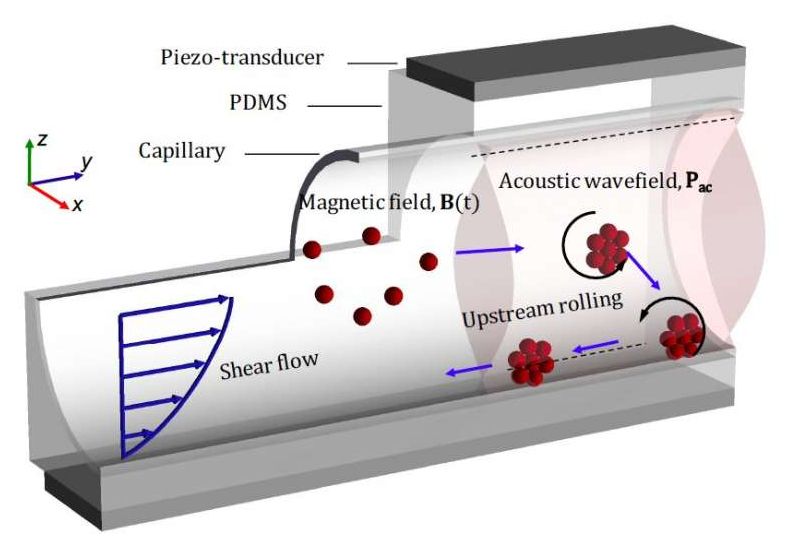
Micro-sized robots could bring a new wave of innovation in the medical field by allowing doctors to access specific regions inside the human body without the need for highly invasive procedures. Among other things, these tiny robots could be used to carry drugs, genes or other substances to specific sites inside the body, opening up new possibilities for treating different medical conditions.
Researchers at ETH Zurich and Helmholtz Institute Erlangen–Nürnberg for Renewable Energy have recently developed micro and nano-sized robots inspired by biological micro-swimmers (e.g., bacteria or spermatozoa). These small robots, presented in a paper published in Nature Machine Intelligence, are capable of upstream motility, which essentially means that they can autonomously move in the opposite direction to that in which a fluid (e.g., blood) flows. This makes them particularly promising for intervening inside the human body.
“We believe that the ideas discussed in our multidisciplinary study can transform many aspects of medicine by enabling tasks such as targeted and precise delivery of drugs or genes, as well as facilitating non-invasive surgeries,” Daniel Ahmed, lead author of the recent paper, told TechXplore.
Self-assembly is ubiquitous in the natural world, serving as a route to form organized structures in every living organism. This phenomenon can be seen, for instance, when two strands of DNA—without any external prodding or guidance—join to form a double helix, or when large numbers of molecules combine to create membranes or other vital cellular structures. Everything goes to its rightful place without an unseen builder having to put all the pieces together, one at a time.
For the past couple of decades, scientists and engineers have been following nature’s lead, designing molecules that assemble themselves in water, with the goal of making nanostructures, primarily for biomedical applications such as drug delivery or tissue engineering. “These small-molecule-based materials tend to degrade rather quickly,” explains Julia Ortony, assistant professor in MIT’s Department of Materials Science and Engineering (DMSE), “and they’re chemically unstable, too. The whole structure falls apart when you remove the water, particularly when any kind of external force is applied.”
She and her team, however, have designed a new class of small molecules that spontaneously assemble into nanoribbons with unprecedented strength, retaining their structure outside of water. The results of this multi-year effort, which could inspire a broad range of applications, were described on Jan. 21 in Nature Nanotechnology by Ortony and coauthors.
Australian scientists have discovered a new way to analyze microscopic cells, tissues and other transparent specimens, through the improvement of an almost 100-year-old imaging technique.
La Trobe University researchers have led a four-year collaboration to make “the invisible visible” by using custom-designed nanomaterials to enhance the sensitivity of phase contrast microscopy, an imaging technique commonly used by scientists to study biological specimens.
The discovery, detailed in Nature Photonics, will benefit a broad range of researchers and has the potential to advance research into the understanding and detection of disease.
Laser beams can be used to change the properties of materials in an extremely precise way. This principle is already widely used in technologies such as rewritable DVDs. However, the underlying processes generally take place at such unimaginably fast speeds and at such a small scale that they have so far eluded direct observation. Researchers at the University of Göttingen and the Max Planck Institute (MPI) for Biophysical Chemistry in Göttingen have now managed to film, for the first time, the laser transformation of a crystal structure with nanometre resolution and in slow motion in an electron microscope. The results have been published in the journal Science.
The team, which includes Thomas Danz and Professor Claus Ropers, took advantage of an unusual property of a material made up of atomically thin layers of sulfur and tantalum atoms. At room temperature, its crystal structure is distorted into tiny wavelike structures—a “charge-density wave” is formed. At higher temperatures, a phase transition occurs in which the original microscopic waves suddenly disappear. The electrical conductivity also changes drastically, an interesting effect for nano-electronics.
In their experiments, the researchers induced this phase transition with short laser pulses and recorded a film of the charge-density wave reaction. “What we observe is the rapid formation and growth of tiny regions where the material was switched to the next phase,” explains first author Thomas Danz from Göttingen University. “The ultrafast transmission electron microscope developed in Göttingen offers the highest time resolution for such imaging in the world today.” The special feature of the experiment lies in a newly developed imaging technique, which is particularly sensitive to the specific changes observed in this phase transition. The Göttingen physicists use it to take images that are composed exclusively of electrons that have been scattered by the crystal’s waviness.
Forever we have held a view that AGING, DISEASE & DEATH is an un-alterable eventuality, those who dared question were ostracised for playing God.
If you choose to look deeper you will surely be amazed. Bowhead whales live for over 200 yrs “Turriptosis Dohnri” is a Jellyfish that lives forever. Can these #genetics traits be replicated in humans? Could the removal of #senescence #cells that accelerates aging be the answer Is it even possible to control or reverse aging? Can we grow old healthily? 150000 die every day & over 100000 of them are caused by aging.
Catch Joao Pedro de Magalhaes microbiologist at Centaura & founder at Magellan Science Ltd. share his insights on the science of #humanlongevity #gerontology.
Change Transform INDIA-CHANGE I M POSSIBLE is a podcast & a platform for the brave Disruptors who don’t conform to the convention. subscribe, support & share India’s 1st #futuretech #podcast #agereversal #reverseaging #longevity #immortality #science
Joao Pedro Magalhaes is a Professor at the University of Liverpool in England.
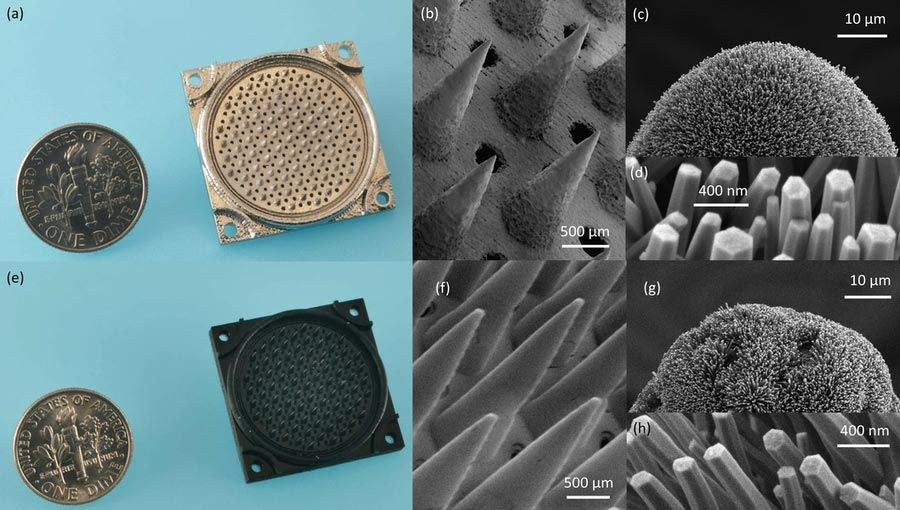
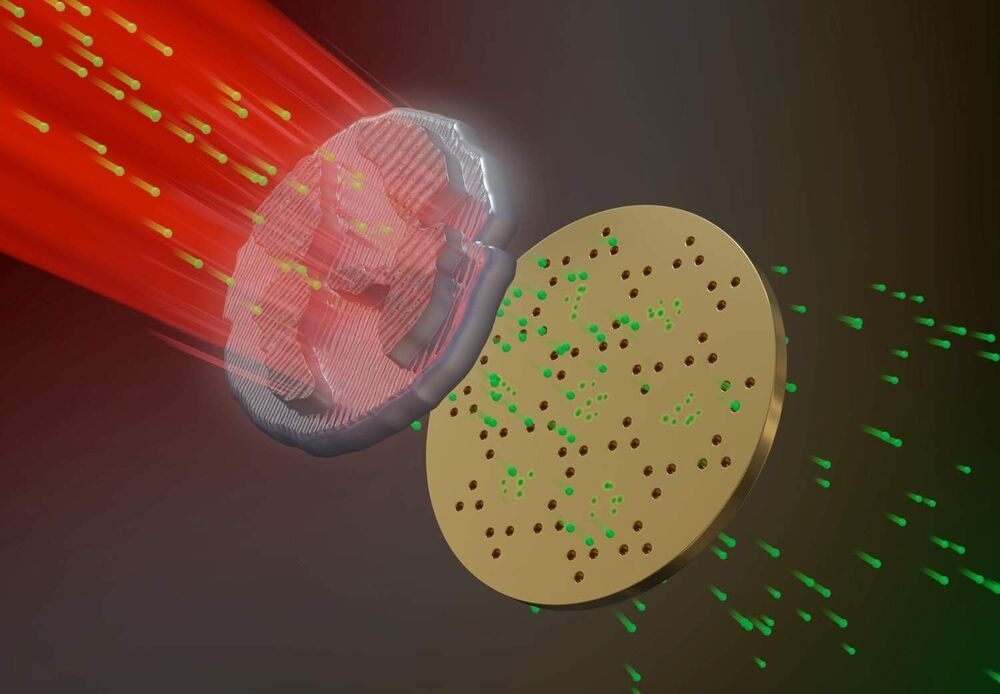
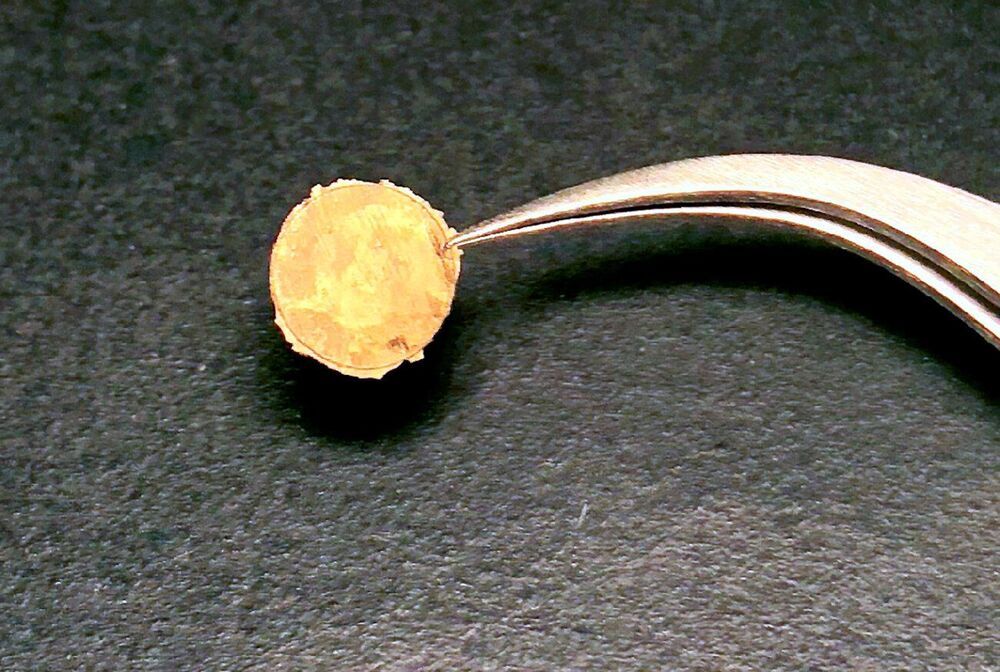
Study is first demonstration of a fully 3D-printed thruster using pure ion emission for propulsion.
A 3D-printed thruster that emits a stream of pure ions could be a low-cost, extremely efficient propulsion source for miniature satellites.
The nanosatellite thruster created by MIT researchers is the first of its kind to be entirely additively manufactured, using a combination of 3D printing and hydrothermal growth of zinc oxide nanowires. It is also the first thruster of this type to produce pure ions from the ionic liquids used to generate propulsion.
Metallurgists have all kinds of ways to make a chunk of metal harder. They can bend it, twist it, run it between two rollers or pound it with a hammer. These methods work by breaking up the metal’s grain structure—the microscopic crystalline domains that form a bulk piece of metal. Smaller grains make for harder metals.
Now, a group of Brown University researchers has found a way to customize metallic grain structures from the bottom up. In a paper published in the journal Chem, the researchers show a method for smashing individual metal nanoclusters together to form solid macro-scale hunks of solid metal. Mechanical testing of the metals manufactured using the technique showed that they were up to four times harder than naturally occurring metal structures.
“Hammering and other hardening methods are all top-down ways of altering grain structure, and it’s very hard to control the grain size you end up with,” said Ou Chen, an assistant professor of chemistry at Brown and corresponding author of the new research. “What we’ve done is create nanoparticle building blocks that fuse together when you squeeze them. This way we can have uniform grain sizes that can be precisely tuned for enhanced properties.”