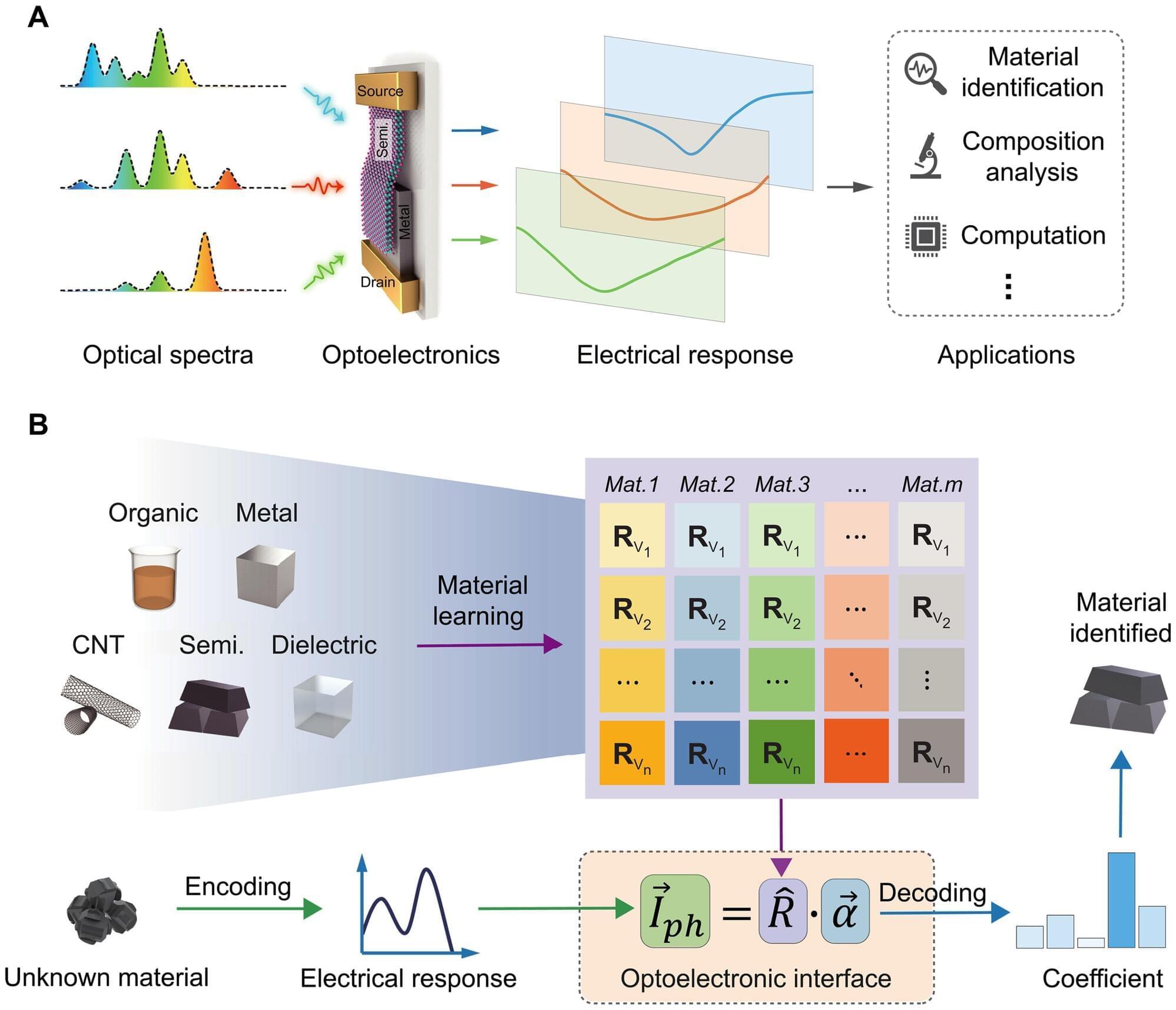
Imagine smartphones that can diagnose diseases, detect counterfeit drugs or warn of spoiled food. Spectral sensing is a powerful technique that identifies materials by analyzing how they interact with light, revealing details far beyond what the human eye can see.
Traditionally, this technology required bulky, expensive systems confined to laboratories and industrial applications. But what if this capability could be miniaturized to fit inside a smartphone or wearable device?
Researchers at Aalto University in Finland have combined miniaturized hardware and intelligent algorithms to create a powerful tool that is compact, cost-effective, and capable of solving real-world problems in areas such as health care, food safety and autonomous driving. The research is published in the journal Science Advances.