2Institute for Lung Health, Cardiopulmonary Institute, Member of German Lung Center, Justus-Liebig University, Giessen, Germany.
3Division of Pharmacology, Otto Loewi Research Center, Medical University of Graz, Graz, Austria.

Mixed urinary incontinence presents a clinical conundrum. Patients with mixed urinary incontinence report symptoms of both stress incontinence (loss of urine with exertion) and urge incontinence (loss of urine with urgency). Mixed urinary incontinence is a combination of the two that affects 37% of women older than age 65 years.1 The personal and societal costs of incontinence are significant. In women with symptoms of severe urinary incontinence, the cost of supplies, laundry, and dry cleaning range from $900 to $4000 annually.2 By 80 years of age, 20% of women will undergo surgery for stress or mixed urinary incontinence.3 Physical and behavioral therapy improves both incontinence types, and medications are standard treatment for urgency urinary incontinence. When conservative therapies fail, conventional guidance has been to treat the urgency prior to the stress component of mixed incontinence, because anti-incontinence surgical procedures can worsen urgency incontinence, and many urgency treatments are medical rather than surgical.4-7 Another strategy has been to treat whichever symptom is dominant.8
A previously published randomized trial of patients with mixed urinary incontinence compared midurethral sling plus behavioral and physical therapy vs sling alone. Findings from the Effects of Surgical Treatment Enhanced With Exercise for Mixed Urinary Incontinence (ESTEEM) trial revealed that both groups, with or without behavioral and physical therapy, reported improved urgency symptoms, findings that substantiated prior cohort studies.9-11 While the original hypothesis of ESTEEM was that treating both components of mixed urinary incontinence with behavioral and physical therapy plus sling would result in better patient outcomes, ESTEEM revealed that urgency symptoms can improve with the midurethral sling alone, challenging previously held beliefs about the impact of anti-incontinence surgeries worsening the urgency component of mixed incontinence.
In this issue of JAMA, investigators report the results of an important trial that is the next natural step in exploring how best to treat mixed urinary incontinence.12 The Treatment for Mixed Urinary Incontinence: Midurethral Sling vs Botox A (MUSA) is a randomized clinical trial of 137 patients with mixed urinary incontinence and moderate bother from both stress and urge symptoms randomized to either the midurethral sling or 100 U of onabotulinumtoxinA.12 Participants had an average number of 7 leakage episodes a day, representing patients severely affected by incontinence. Importantly, patients previously had unsuccessful conservative interventions, including medications. The investigators hypothesized that treating the urgency component of mixed urinary incontinence with onabotulinumtoxinA would result in better outcomes than focusing on the stress component with a midurethral sling.
Imagine getting a tattoo… that can track your health, location, or identity — and you don’t even need a device. Sounds like sci-fi? It’s real. Scientists have developed futuristic electronic tattoos that use special ink to monitor your body in real-time — from heart rate to hydration — and even transmit data without chips or batteries. But here’s the catch… could this breakthrough be the future of medicine? Or is it a step too close to surveillance under your skin?
Let’s explore how these tattoos work, what they can really do, and the wild implications they might have for your health — and your privacy.
🔔 Subscribe now for more mind-blowing science and tech stories!
Credit:
ScienceVio / YouTube.
Seeker / YouTube.
CUHK Engineering / YouTube.
NewsNation / YouTube.
Animation is created by Bright Side.
Music from TheSoul Sound: https://thesoul-sound.com/
This high energy output could vastly improve the world’s sustainability. With fusion, energy would be near-limitless and thus easily accessible and substantially more affordable. People could enjoy lower utility bills and consistent, reliable energy.
Watch now: How bad is a gas stove for your home’s indoor air quality?
The innovative reactor would help slow down climate change and lead to a cleaner, cooler future, while helping people save money and access clean energy. Reducing energy pollution will benefit every human, reducing the health hazards of breathing polluted air or drinking contaminated water.
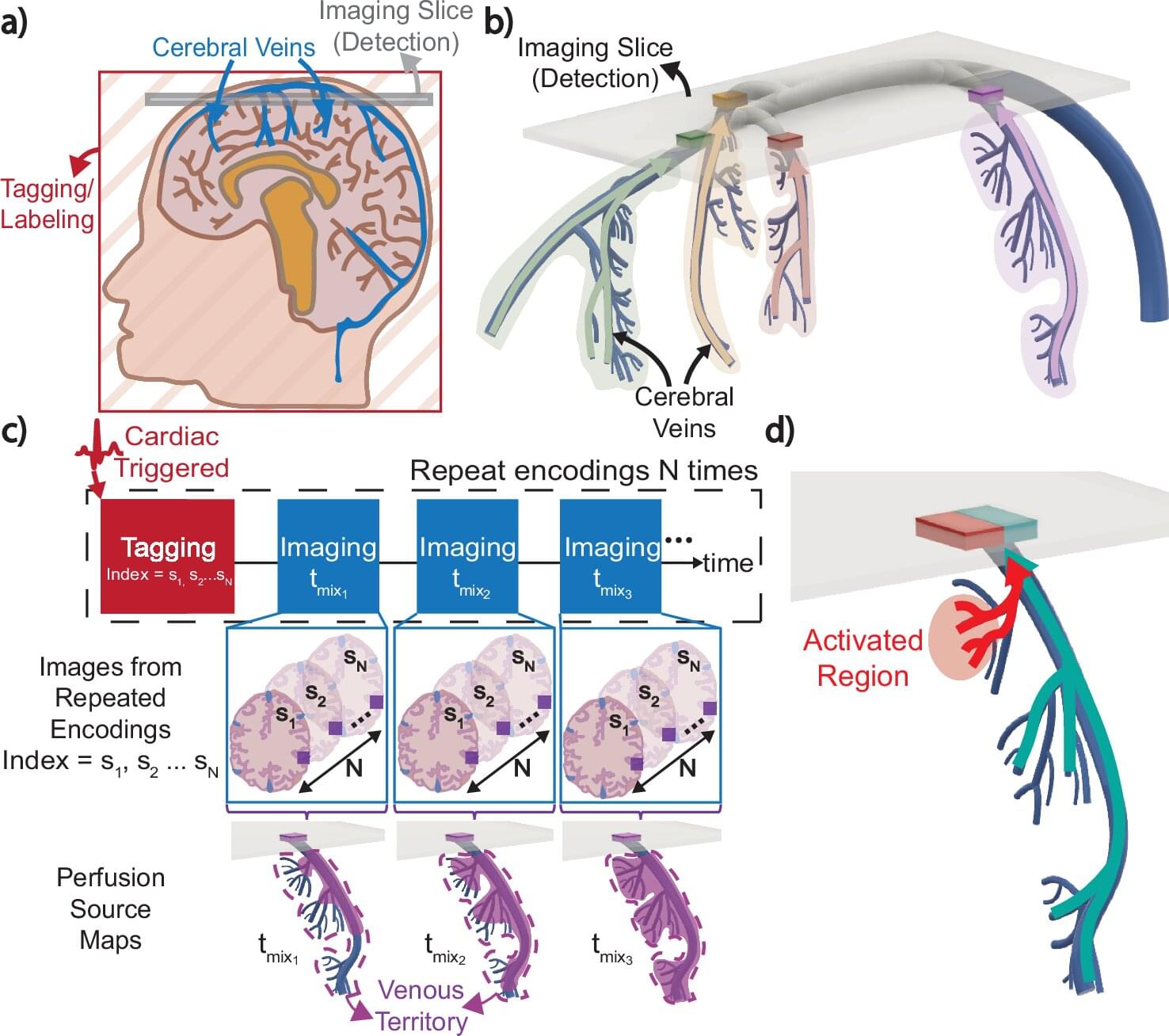
The venous system maintains the health of our brains by removing deoxygenated blood and other waste products, but its complexity and variability have made scientific study difficult. Now, a UC Berkeley-led team of researchers has developed an innovative MRI technique that may expand our understanding of this critical system.
In a study published in Nature Communications, the researchers demonstrate how their new imaging method, Displacement Spectrum (DiSpect) MRI, maps blood flows “in reverse” to reveal the source of blood in the brain’s veins. This approach could help answer long-standing questions about brain physiology as well as provide a safer, more efficient way to diagnose disease.
Like some current MRI methods, DiSpect uses the water in our blood as a tracing agent to map perfusion, or blood flow, in the brain. The water’s hydrogen atoms possess a quantum mechanical property called spin and can be magnetized when exposed to a magnetic field, like an MRI scanner. But what makes DiSpect unique is its ability to track the “memory” of these nuclear spins, allowing it to map blood flow back to its source.
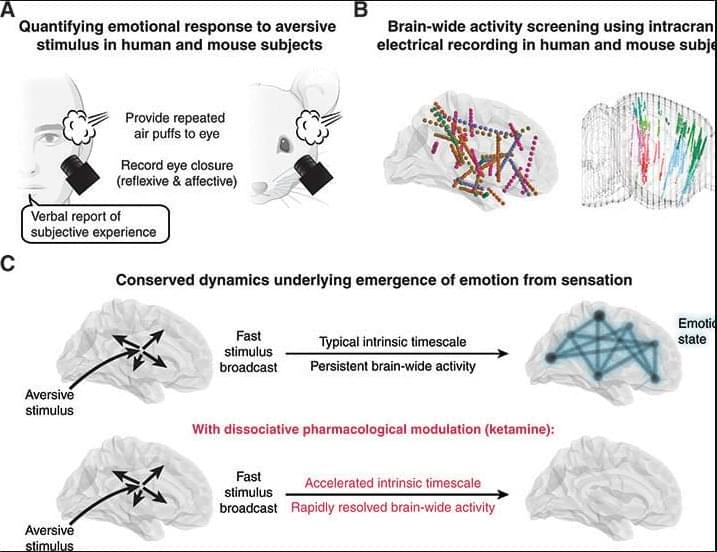
Emotional responses to sensory experience are central to the human condition in health and disease. We hypothesized that principles governing the emergence of emotion from sensation might be discoverable through their conservation across the mammalian lineage. We therefore designed a cross-species neural activity screen, applicable to humans and mice, combining precise affective behavioral measurements, clinical medication administration, and brain-wide intracranial electrophysiology. This screen revealed conserved biphasic dynamics in which emotionally salient sensory signals are swiftly broadcast throughout the brain and followed by a characteristic persistent activity pattern. Medication-based interventions that selectively blocked persistent dynamics while preserving fast broadcast selectively inhibited emotional responses in humans and mice.


In this episode of The Moss Report, Ben Moss sits down with Dr. Ralph Moss to explore the real-world pros and cons of using artificial intelligence in cancer research and care.
From AI-generated health advice to PubMed citations that don’t exist, this honest conversation covers what AI tools are getting right—and where they can dangerously mislead.
Dr. Moss shares the results of his own AI test across five major platforms, exposing their strengths and surprising failures.
Whether you’re a cancer patient, caregiver, or simply curious about how AI is shaping the future of medicine, this episode is essential listening.
Links and Resources:
🌿 The Moss Method – Fight Cancer Naturally – (Paperback, Hardcover, Kindle) https://amzn.to/4dGvVjp.