Interior minister says ammonium nitrate likely caused at least one explosion amid reports hospitals too damaged to treat patients.



A disease-detecting “precision health” toilet can sense multiple signs of illness through automated urine and stool analysis, according to a new study.
The “smart toilet” isn’t the kind that lifts its own lid in preparation for use; this toilet includes technology that can detect a range of disease markers in stool and urine, including those of some cancers, such as colorectal or urologic cancers.
The device could hold particular appeal for people genetically predisposed to certain conditions, such as irritable bowel syndrome, prostate cancer, or kidney failure, and want to keep on top of their health.
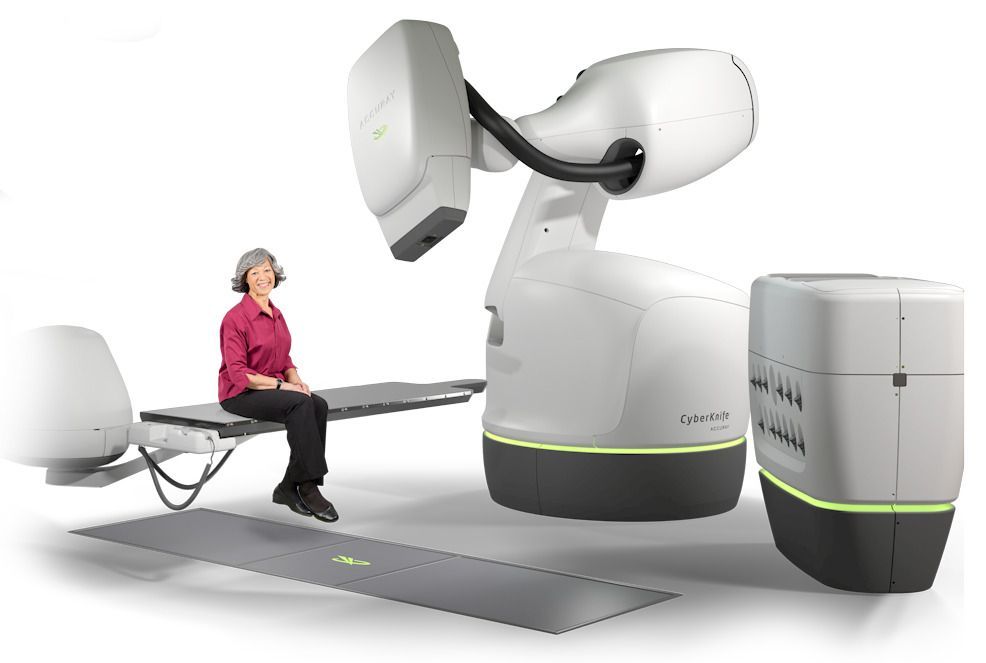
February 27, 2020 — Accuray Incorporated announced that Mercy Hospital St. Louis continues to demonstrate its commitment to improving patient outcomes with the installation of the first CyberKnife M6 System in Missouri at their state-of-the-art David C. Pratt Cancer Center. The next-generation CyberKnife System has been shown to deliver precise stereotactic radiosurgery (SRS) and stereotactic body radiation therapy (SBRT) treatments with ease, making it possible for the clinical team to expand access to one of the most advanced methods for administering radiation to more cancer patients.
SRS and SBRT are non-invasive forms of radiation therapy that use high doses of very targeted radiation to destroy tumors, in just a few treatment sessions (1 to 5). SRS is commonly used to treat conditions within the brain and spine, while SBRT is used for those tumors located outside these areas. The CyberKnife M6 System is equipped with sophisticated functionality that will streamline the creation of personalized treatment plans and reduce the time to deliver radiation treatments, enabling the Mercy St. Louis team to offer precision SRS and SBRT treatments to more patients each day.
The Mercy Hospital St. Louis team uses the most advanced radiotherapy technology to design and deliver an individualized treatment plan designed to help cancer patients take control of their disease and resume their lives. The hospital is part of the Mercy system, named one of the top five large U.S. health systems from 2016 to 2019 by IBM Watson Health. Mercy announced in 2018 that it intended to work with Accuray to enhance cancer care through advanced life-saving technology, including the CyberKnife System that was recently installed as well as Accuray Radixact Systems that will be installed at other Mercy hospitals.

The COVID-19 pandemic didn’t just transform how we work and communicate. It also accelerated the need for more proactive health measures for chronic health problems tied to diet. Such problems have emerged as a top risk factor for coronavirus and people with poor metabolic health accounted for half of COVID-19 hospitalizations in some regions around the world. The resulting high numbers led the authors of a report in The Lancet to issue a call for more resources to tackle metabolic health to avoid needless deaths.
Thankfully, new tools have been developed to offer comprehensive understanding of nutrition. This expertise and technology won’t just help us tackle metabolic health – it could help us finally fully realize the power of plants to improve health and wellness outcomes.
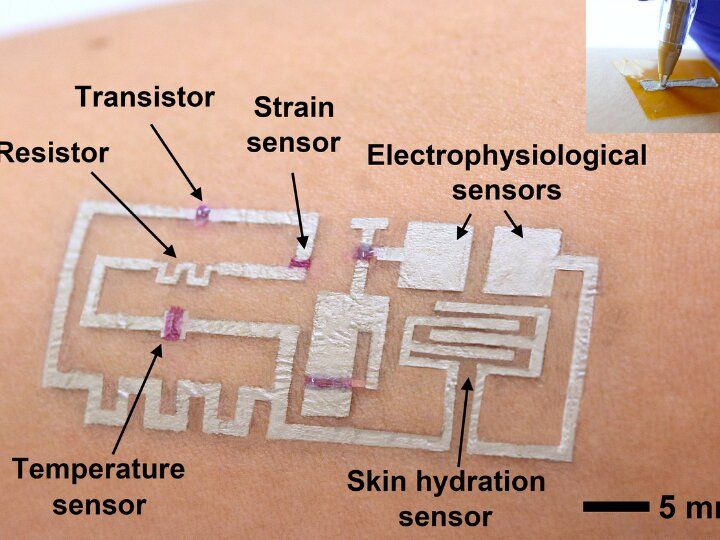
A team of researchers led by Cunjiang Yu, Bill D. Cook Associate Professor of Mechanical Engineering at the University of Houston, has developed a new form of electronics known as “drawn-on-skin electronics,” allowing multifunctional sensors and circuits to be drawn on the skin with an ink pen.
The advance, the researchers report in Nature Communications, allows for the collection of more precise, motion artifact-free health data, solving the long-standing problem of collecting precise biological data through a wearable device when the subject is in motion.
The imprecision may not be important when your FitBit registers 4,000 steps instead of 4,200, but sensors designed to check heart function, temperature and other physical signals must be accurate if they are to be used for diagnostics and treatment.
ENCODE Project’s third phase offers new insights into the organization and regulation of our genes and genome.
The Encyclopedia of DNA Elements (ENCODE) Project is a worldwide effort to understand how the human genome functions. With the completion of its latest phase, the ENCODE Project has added millions of candidate DNA “switches” from the human and mouse genomes that appear to regulate when and where genes are turned on, and a new registry that assigns a portion of these DNA switches to useful biological categories. The project also offers new visualization tools to assist in the use of ENCODE’s large datasets.
The project’s latest results were published in Nature, accompanied by 13 additional in-depth studies published in other major journals. ENCODE is funded by the National Human Genome Research Institute, part of the National Institutes of Health.
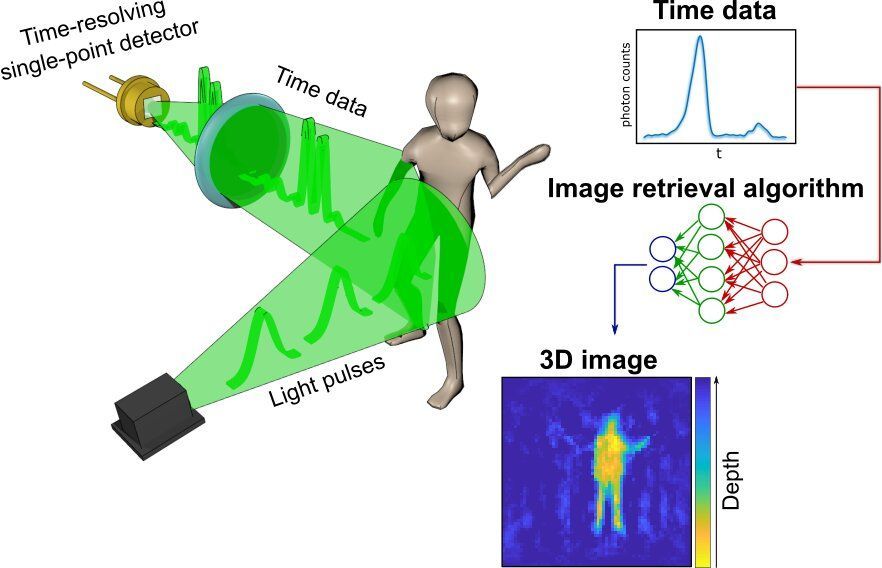
A radical new method of imaging that harnesses artificial intelligence to turn time into visions of 3D space could help cars, mobile devices and health monitors develop 360-degree awareness.
Photos and videos are usually produced by capturing photons—the building blocks of light—with digital sensors. For instance, digital cameras consist of millions of pixels that form images by detecting the intensity and color of the light at every point of space. 3D images can then be generated either by positioning two or more cameras around the subject to photograph it from multiple angles, or by using streams of photons to scan the scene and reconstruct it in three dimensions. Either way, an image is only built by gathering spatial information of the scene.
In a new paper published today in the journal Optica, researchers based in the U.K., Italy and the Netherlands describe an entirely new way to make animated 3D images: by capturing temporal information about photons instead of their spatial coordinates.


Dowload the pdf
Cannabis sativa, especially one high in the anti-inflammatory cannabinoid cannabidiol (CBD), has been proposed to modulate gene expression and inflammation and harbour anti-cancer and anti-inflammatory properties. Working under the Health Canada research license, we have developed over 800 new Cannabis sativa lines and extracts and hypothesized that high-CBD C. sativa extracts may be used to modulate ACE2 expression in COVID-19 target tissues. Screening C. sativa extracts using artificial human 3D models of oral, airway, and intestinal tissues, we identified 13 high CBD C. sativa extracts that modulate ACE2 gene expression and ACE2 protein levels. Our initial data suggest that some C. sativa extract down-regulate serine protease TMPRSS2, another critical protein required for SARS-CoV2 entry into host cells.
While our most effective extracts require further large-scale validation, our study is crucial for the future analysis of the effects of medical cannabis on COVID-19. The extracts of our most successful and novel high CBD C. sativa lines, pending further investigation, may become a useful and safe addition to the treatment of COVID-19 as an adjunct therapy. They can be used to develop easy-to-use preventative treatments in the form of mouthwash and throat gargle products for both clinical and at-home use. Such products ought to be tested for their potential to decrease viral entry via the oral mucosa. Given the current dire and rapidly evolving epidemiological situation, every possible therapeutic opportunity and avenue must be considered.
With the rapidly growing pandemic of COVID-19 caused by the new and challenging to treat zoonotic SARS-CoV2 coronavirus, there is an urgent need for new therapies and prevention strategies that can help curtail disease spread and reduce mortality. Inhibition of viral entry and thereby spread constitute plausible therapeutic avenues. Similar to other respiratory pathogens, SARS-CoV2 is transmitted through respiratory droplets, with potential for aerosol and contact spread. It uses receptor-mediated entry into the human host via angiotensin-converting enzyme II (ACE2) that is expressed in lung tissue, as well as oral and nasal mucosa, kidney, testes, and the gastrointestinal tract. Modulation of ACE2 levels in these gateway tissues may prove a plausible strategy for decreasing disease susceptibility. Cannabis sativa, especially one high in the anti-inflammatory cannabinoid cannabidiol (CBD), has been proposed to modulate gene expression and inflammation and harbour anti-cancer and anti-inflammatory properties. Working under the Health Canada research license, we have developed over 800 new Cannabis sativa lines and extracts and hypothesized that high-CBD C. sativa extracts may be used to modulate ACE2 expression in COVID-19 target tissues. Screening C. sativa extracts using artificial human 3D models of oral, airway, and intestinal tissues, we identified 13 high CBD C. sativa extracts that modulate ACE2 gene expression and ACE2 protein levels. Our initial data suggest that some C. sativa extract down-regulate serine protease TMPRSS2, another critical protein required for SARS-CoV2 entry into host cells. While our most effective extracts require further large-scale validation, our study is crucial for the future analysis of the effects of medical cannabis on COVID-19. The extracts of our most successful and novel high CBD C. sativa lines, pending further investigation, may become a useful and safe addition to the treatment of COVID-19 as an adjunct therapy. They can be used to develop easy-to-use preventative treatments in the form of mouthwash and throat gargle products for both clinical and at-home use. Such products ought to be tested for their potential to decrease viral entry via the oral mucosa. Given the current dire and rapidly evolving epidemiological situation, every possible therapeutic opportunity and avenue must be considered.

This sucks.
Per- and polyfluoroalkyl substances (PFAS), found in many household products and food packages, have raised concerns because of their persistence and possible toxicity to people and wildlife. Because the compounds don’t break down naturally, they have become environmental contaminants. Now, researchers reporting in Environmental Science & Technology have studied the transport of 29 PFAS into and out of the Arctic Ocean, detecting a newer compound for the first time in Arctic seawater.
After studies indicated that two PFAS—PFOA and PFOS—can cause cancer, a compromised immune response and other health problems in lab animals, the two compounds were voluntarily phased out by industry. However, these legacy compounds are still widely detected in the environment. Intended as a safer replacement for PFOA, HFPO-DA (sold under the trade name GenX) is now thought to pose similar health and persistence concerns. Hanna Joerss and colleagues wanted to investigate the long-range, oceanic transport of legacy and replacement PFAS to the Arctic Ocean—a remote body of water connected to the Atlantic Ocean by the Fram Strait, which is located between Svalbard and Greenland.
Aboard an icebreaker research ship, the team collected water samples along two Fram Strait currents entering and exiting the Arctic Ocean and along a path from Europe’s North Sea to the Arctic Ocean. Using mass spectrometry, the researchers detected 11 PFAS in the ocean water, including PFOA, HFPO-DA and other long- and short-chain PFAS. This was the first time that HFPO-DA had been detected in seawater from a remote region, indicating that the compound can be transported long distances. Higher levels of PFAS were detected in the water exiting the Arctic Ocean compared with the water entering the Arctic from the North Atlantic. The PFAS composition in the outgoing water suggested that more of these compounds arose from atmospheric sources than from ocean circulation.