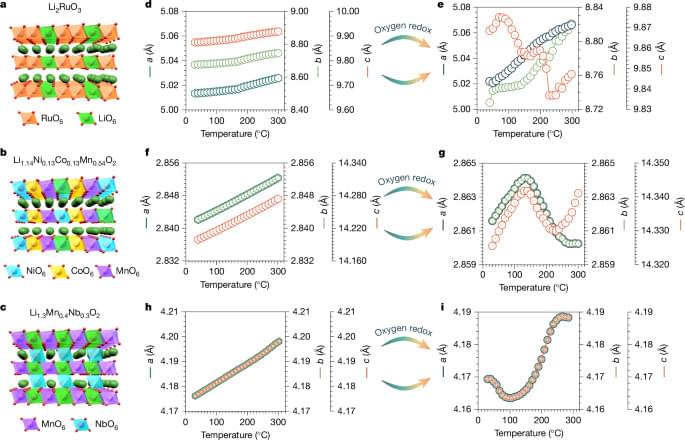
Northeastern University researchers resurrected an extinct plant gene, turning back the evolutionary clock to pave a path forward for the development and discovery of new drugs.
Specifically, the team, led by Jing-Ke Weng, a professor of chemistry, chemical biology and bioengineering at Northeastern, repaired a defunct gene in the coyote tobacco plant.
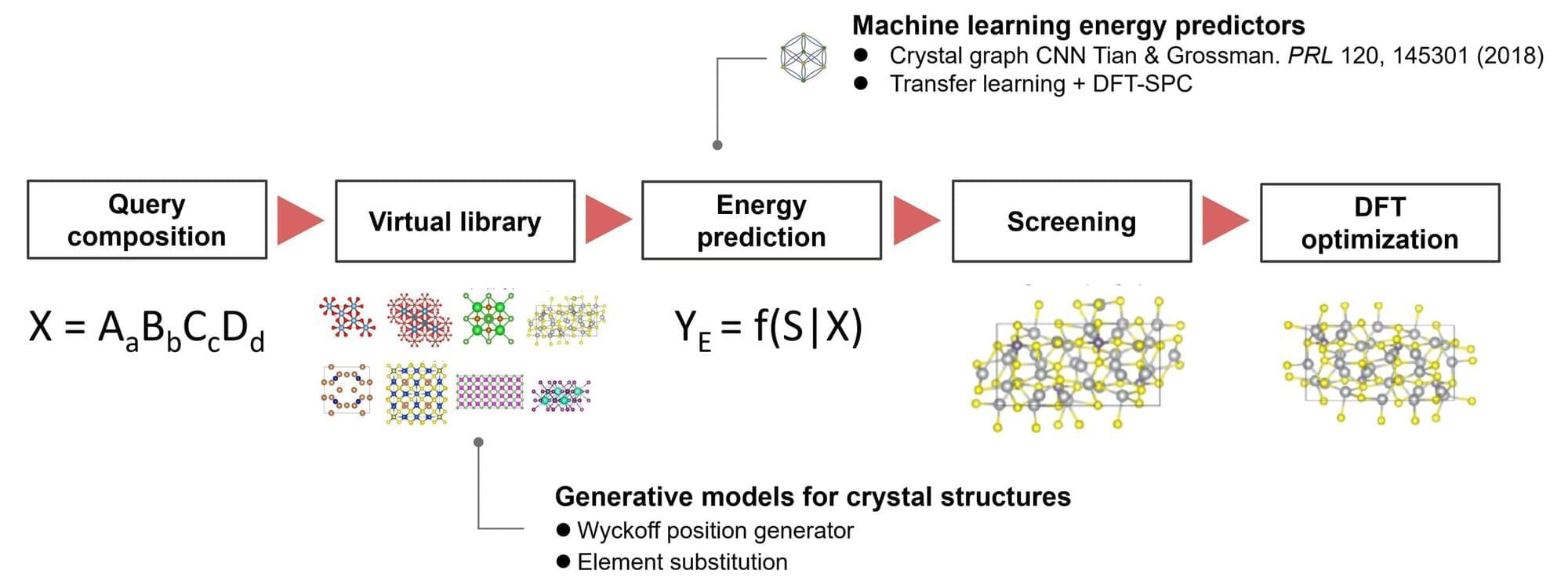
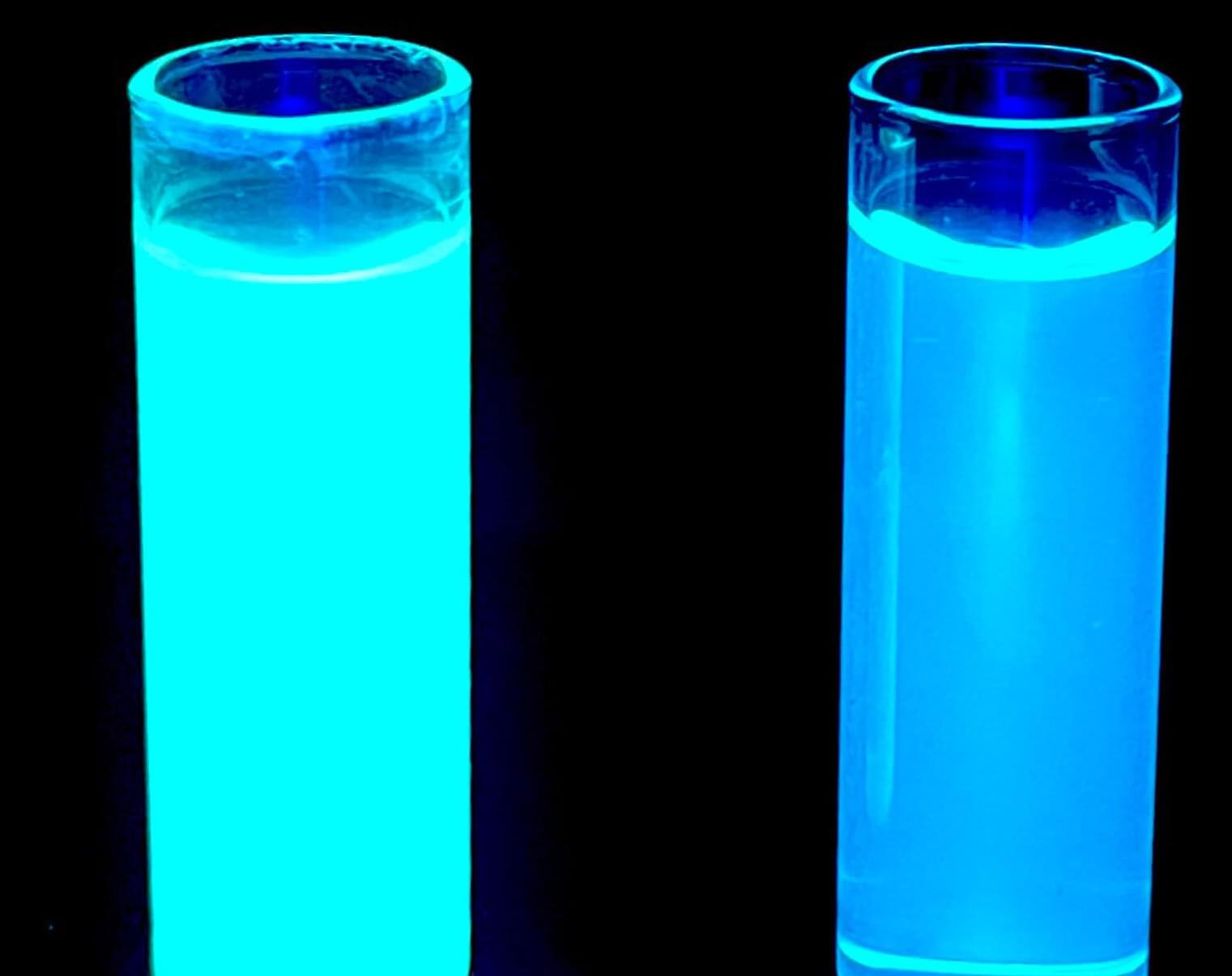
In a new paper, they detail their discovery of a previously unknown kind of cyclic peptide, or mini-protein, called nanamin that is easy to bioengineer, making it “a platform with huge potential for drug discovery,” Weng says. The paper is published in the journal Proceedings of the National Academy of Sciences.