The basics for tracking include blood biomarkers that have been studied for 50 – 100+ years, depending on the biomarker. Most of these biomarkers are commonly measured at a yearly physical, and are relatively cheap (35 $USD for the standard chemistry panel and complete blood count).
Category: chemistry – Page 365
DeepMind Simulates Matter on the Nanoscale With Artificial Intelligence
In a paper published by Science, DeepMind demonstrates how neural networks can improve approximation of the Density Functional (a method used to describe electron interactions in chemical systems). This illustrates deep learning’s promise in accurately simulating matter at the quantum mechanical.
In a paper published in the scientific journal Science, DeepMind demonstrates how neural networks can be used to describe electron interactions in chemical systems more accurately than existing methods.
Density Functional Theory, established in the 1960s, describes the mapping between electron density and interaction energy. For more than 50 years, the exact nature of mapping between electron density and interaction energy — the so-called density functional — has remained unknown. In a significant advancement for the field, DeepMind has shown that neural networks can be used to build a more accurate map of the density and interaction between electrons than was previously attainable.
By expressing the functional as a neural network and incorporating exact properties into the training data, DeepMind was able to train the model to learn functionals free from two important systematic errors — the delocalization error and spin symmetry breaking — resulting in a better description of a broad class of chemical reactions.
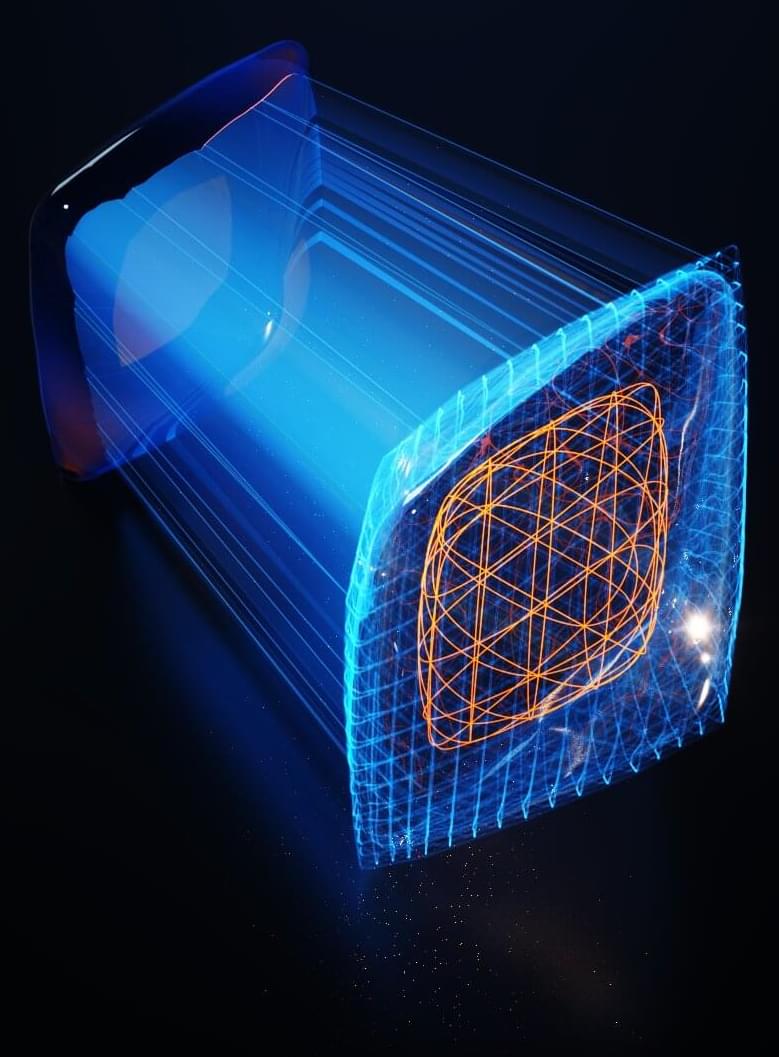
Scientists combine AI and atomic-scale images in pursuit of better batteries
Today’s rechargeable batteries are a wonder, but far from perfect. Eventually, they all wear out, begetting expensive replacements and recycling.
“But what if batteries were indestructible?” asks William Chueh, an associate professor of materials science and engineering at Stanford University and senior author of a new paper detailing a first-of-its-kind analytical approach to building better batteries that could help speed that day. The study appears in the journal Nature Materials.
Chueh, lead author Haitao “Dean” Deng, Ph.D. ‘21, and collaborators at Lawrence Berkeley National Laboratory, MIT and other research institutions used artificial intelligence to analyze new kinds of atomic-scale microscopic images to understand exactly why batteries wear out. Eventually, they say, the revelations could lead to batteries that last much longer than today’s. Specifically, they looked at a particular type of lithium-ion batteries based on so-called LFP materials, which could lead to mass-market electric vehicles because it does not use chemicals with constrained supply chains.

Researchers create molecule that can pave way for mini-transistors
Researchers at Lund University in Sweden have succeeded in developing a simple hydrocarbon molecule with a logic gate function, similar to that in transistors, in a single molecule. The discovery could make electric components on a molecular scale possible in the future. The results are published in Nature Communications.
Manufacturing very small components is an important challenge in both research and development. One example is transistors—the smaller they are, the faster and more energy efficient our computers become. But is there a limit to how small logic gates can become? And is it possible to create electric machines on a molecular scale? Yes, perhaps, is the answer from a chemistry research team at Lund University.
“We have developed a simple hydrocarbon molecule that changes its form, and at the same time goes from insulating to conductive, when exposed to electric potential. The successful formula was to design a so-called anti-aromatic ring in a molecule so that it becomes more robust and can both receive and relay electrons,” says Daniel Strand, chemistry researcher at Lund University.
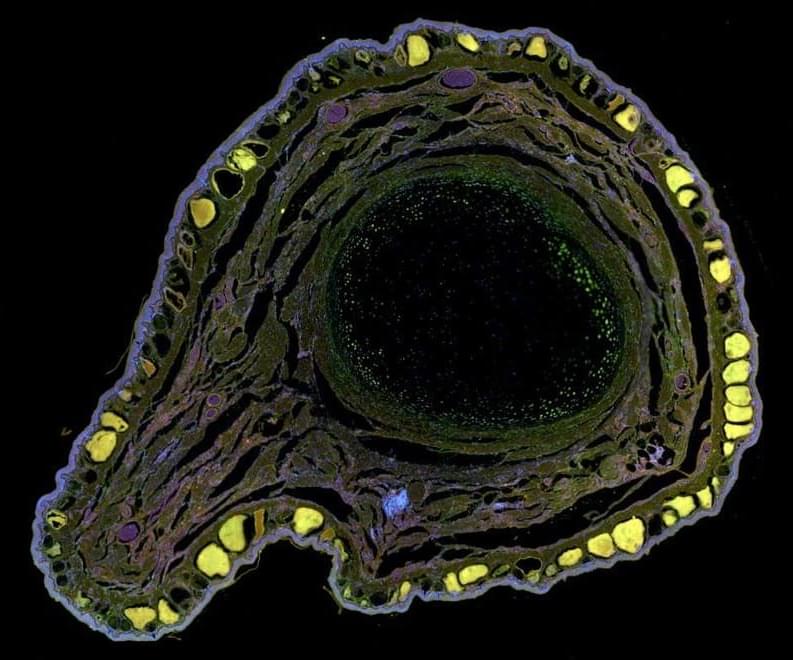
Frogs regrow amputated legs after treatment with a chemical cocktail
Adult frogs can’t usually regrow a lost leg, but they can after treatment with a regenerative cocktail — and the new leg even contains functioning nerves.
Adult frogs can gain the ability to regrow a lost leg if they are treated with a device containing a silk gel infused with five regenerative chemicals. The limbs the frogs grow can apparently move and sense as well as the original legs.
Although tadpoles and young froglets can regenerate hindlimbs, adult frogs, like humans, lack the capacity to regrow their legs.
“We were [looking for] a way to kickstart regeneration in an organism that normally can’t regenerate a limb,” says Nirosha Murugan at Algoma University in Ontario, Canada.

What we knew about water was right after all
A comprehensive investigation by KAUST researchers sets the record straight on the formation of hydrogen peroxide in micrometer-sized water droplets, or microdroplets, and shows that ozone is the key to this transformation1,2.
The air-water interface is a crucial site for numerous natural, domestic and industrial processes such as ocean-atmosphere exchange, cloud and dew formation, aerated beverages and bioreactors. Yet, probing chemical transformations at the air–water interface is challenging due to the lack of surface-specific techniques or computational models.
Recent research revealed that water spontaneously transforms into 30–110 micromolar hydrogen peroxide (H2O2) in microdroplets, obtained by condensing vapor or spraying water using pressurized nitrogen gas. The textbook understanding of water is thus challenged by how the mild temperature and pressure conditions, together with the absence of catalysts, co-solvents and significant applied energy, could break covalent O–H bonds. It was hypothesized that this unusual phenomenon resulted from an ultrahigh electric field at the air-water interface that assists OH radical formation, but no direct evidence has been reported.
Exploration & Origins Colloquium 2022: Space Exploration, Origins, & Astrobiology
The ExplOrigins early career group invites you to join the 2022 Exploration and Origins Colloquium on February 17th–18th, 2022! The live broadcast portion of this colloquium will begin at 10am ET on February 18th.
We are thrilled to have Dr. Amy Mainzer as our plenary speaker. Dr. Mainzer is a professor of planetary science at the University of Arizona, principal investigator of NASA’s Near-Earth Object Wide-field Infrared Survey Explorer (NEOWISE) mission, and lead of NASA’s Near-Earth Object (NEO) Surveyor mission. She also has achieved excellence in science communication, serving as the science curriculum consultant, on-camera host, and executive producer of the PBS Kids series Ready Jet Go! and as the science consultant for the Netflix movie Don’t Look Up.
Our program also highlights current astrobiology, origins, and space science projects presented by a cast of early career researchers from the Atlanta area.
Schedule:
10:00 a.m.
Welcome Speech, Breakfast (Tyler Roche)
10:10 – 11:00 a.m.
Talks Section 1:
Clathrates, Cubesats, and Characterization (Christina Buffo)
Bacterial Clathrate-Binding Proteins in the Deep Subsurface Biosphere: Implications for Gas Clathrate Stability and Habitability (Abigail Johnson)
Virtual Super-Resolution Optics with Reconfigurable Swarms (VISORS): a Two-CubeSat Formation-Flying Telescope for Coronal Observation (William Rawson)
Characterization and Thermal Analysis of Metal Phosphites and Their Role in Astrobiology (Kimberly Faye Meyberg)
11:05 a.m. – 12:00 p.m.

Why the Nuclear Option is a Necessity if Humans Are Ever Going to Get to Mars and Return Alive
The ISS is 1,000 times closer to us than the Moon, and 600,000 times closer than Mars. To get to the latter and back safely, we need faster rocket propulsion systems.
Using the conventional chemical rocket technology we have perfected at this time, a single mission to Mars will require the launch of a mass equal to 10 ISS to be put into space. It will involve at least 30 and as many as 40 of the largest rockets we have today to put the spacecraft, crew and fuel needed for the mission. That doesn’t include adding reserves of fuel placed strategically along the route should a problem arise going to Mars and coming back. Brown states that the total cost of a single mission using this approach would exceed $80 billion using the yet-to-be-launched SLS as the primary vehicle. With SpaceX and the Starship and Heavy booster, the cost could be cut by half. But even $40 billion for a single mission seems excessive.
Using nuclear-powered propulsion systems, however, would eliminate the need to put megatons of fuel into orbit. The only time chemical rockets would be used would be in launching the crew and spaceship components to Earth orbit. That could be done in as few as three launches with the final assembled ship going to Mars and back and then being parked in Earth orbit to be used again on future missions.
What additional advantages can be derived from using nuclear? Thermal-powered nuclear is at least twice as efficient as current chemical rockets and requires a tiny fraction of the fuel to get the job done. Electric-powered nuclear starts moving the rocket slowly away from Earth and then continuously accelerates to attain peak speeds of 200,000 kilometres (120,000 miles) per hour. An electric-powered nuclear propulsion system would use even less fuel than thermal-powered nuclear and would shorten the voyage to a month.
Big Breakthrough for “Massless” Energy Storage: Structural Battery That Performs 10x Better Than All Previous Versions
Researchers from Chalmers University of Technology have produced a structural battery that performs ten times better than all previous versions. It contains carbon fiber that serves simultaneously as an electrode, conductor, and load-bearing material. Their latest research breakthrough paves the way for essentially ’massless’ energy storage in vehicles and other technology.
The batteries in today’s electric cars constitute a large part of the vehicles’ weight, without fulfilling any load-bearing function. A structural battery, on the other hand, is one that works as both a power source and as part of the structure – for example, in a car body. This is termed ‘massless’ energy storage, because in essence the battery’s weight vanishes when it becomes part of the load-bearing structure. Calculations show that this type of multifunctional battery could greatly reduce the weight of an electric vehicle.
The development of structural batteries at Chalmers University of Technology has proceeded through many years of research, including previous discoveries involving certain types of carbon fiber. In addition to being stiff and strong, they also have a good ability to store electrical energy chemically. This work was named by Physics World as one of 2018’s ten biggest scientific breakthroughs.
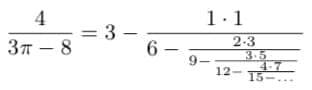
Using algorithms to discover new mathematics
Fundamental constants like e and π are ubiquitous in diverse fields of science, including physics, biology, chemistry, geometry, and abstract mathematics. Nevertheless, for centuries new mathematical formulas relating fundamental constants are scarce and are usually discovered sporadically by mathematical intuition or ingenuity.
Our algorithms search for new mathematical formulas. The community can suggest proofs for the conjectures or even propose or develop new algorithms. Any new conjecture, proof, or algorithm suggested will be named after you.