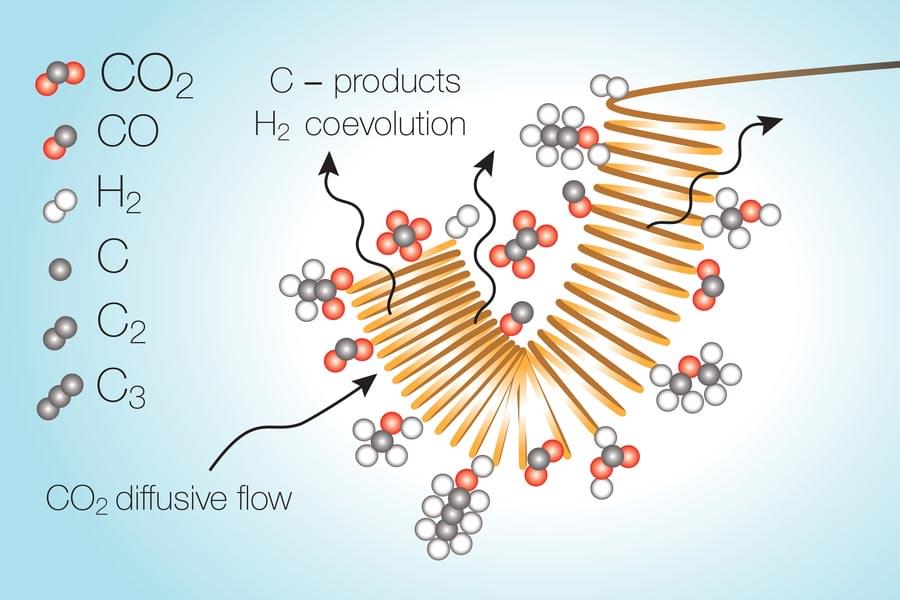
Tech companies continued to draw criticism for their roles in political and social scandals, most notably when whisteblower and former Facebook employee Frances Haugen testified to lawmakers. Undeterred, Facebook rebranded itself Meta and said it would now focus on building the metaverse. Twitter CEO Jack Dorsey stepped down and likewise changed the name of his company Square to Block in a not-so-subtle nod to the blockchain.
Meanwhile, volatile cryptocurrencies set new records, their prices jumping and crashing on a tweet. NFTs, a once-obscure type of cryptoasset, went on an eye-watering tear as redditors pushed meme stocks skyward. It was also the year of ever-bigger AI. Machine learning models surpassed a trillion parameters, designed computer chips, and tackled practical problems in biology, math, and chemistry. Elsewhere, billionaires went to space, regular folks bought 3D printed houses, fusion power attracted billions in investment, gene editing trials hit their stride, and “flying car” companies hit the New York Stock Exchange.
For this year’s list of fascinating stories in tech and science, we sifted our Saturday posts and selected articles that looked back to where it all began, glanced ahead to what’s coming, or otherwise stood out from the chatter to stand the test of time.