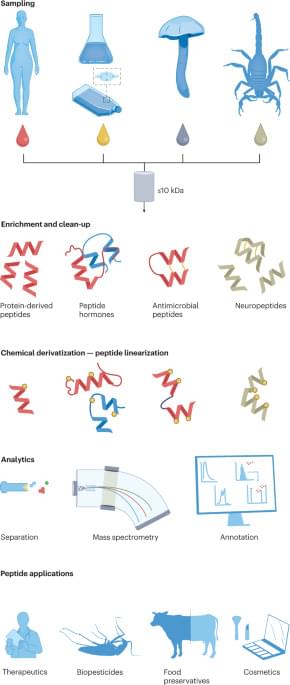
In the last decade, we have witnessed biology bring us some incredible products and technologies: from mushroom-based packaging to animal-free hotdogs and mRNA vaccines that helped curb a global pandemic. The power of synthetic biology to transform our world cannot be overstated: this industry is projected to contribute to as much as a third of the global economic output by 2030, or nearly $30 trillion, and could impact almost every area of our lives, from the food we eat to the medicine we put in our bodies.
The leaders of this unstoppable bio revolution – many of whom you can meet at the SynBioBeta conference in Oakland, CA, on May 23–25 – are bringing the future closer every day through their ambitious vision, long-range strategy, and proactive oversight. These ten powerful women are shaping our world as company leaders, biosecurity experts, policymakers, and philanthropists focused on charting a new course to a more sustainable, equitable, clean, and safe future.
As an early pioneer in the high-throughput synthesis and sequencing of DNA, Emily Leproust has dedicated her life to democratizing gene synthesis to catapult the growth of synthetic biology applications from medicine, food, agriculture, and industrial chemicals to DNA data storage. She was one of the co-founders of Twist Bioscience in 2013 and is still leading the expanding company as CEO. To say that Twist’s silicon platform was a game-changer for the industry is an understatement. And it is no surprise that Leproust was recently honored with the BIO Rosalind Franklin Award for her work in the biobased economy and biotech innovation.