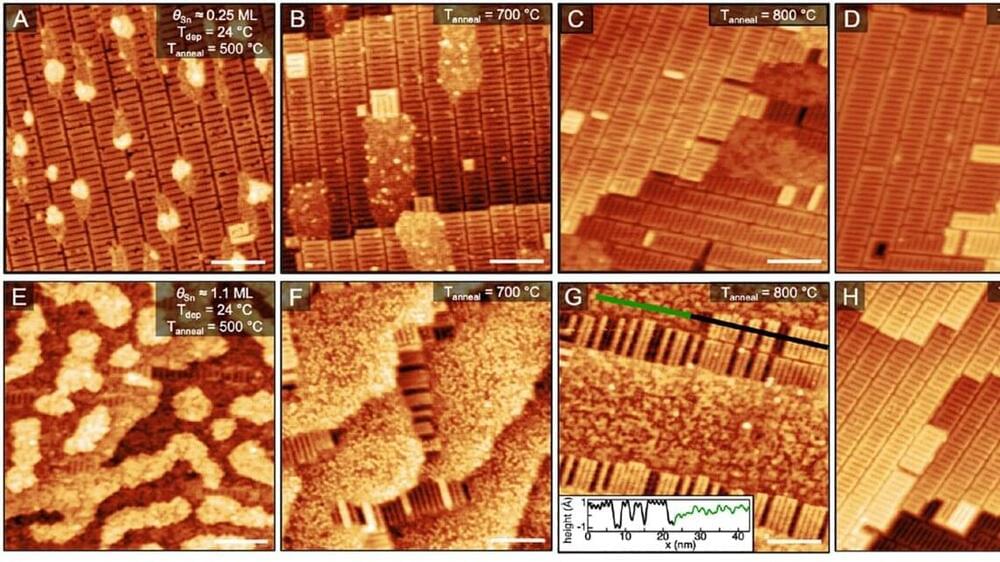
New research from a team of scientists at the Cornell University Center for Bright Beams has made significant strides in developing new techniques to guide the growth of materials used in next-generation particle accelerators.
The study, published in the Journal of Physical Chemistry C, reveals the potential for greater control over the growth of superconducting Nb3Sn films, which could significantly reduce the cost and size of cryogenic infrastructure required for superconducting technology.
Superconducting accelerator facilities, such as those used for X-ray free-electron laser radiation, rely on niobium superconducting radio frequency (SRF) cavities to generate high-energy beams. However, the associated cryogenic infrastructure, energy consumption, and operating costs of niobium SRF cavities limit access to this technology.