Carbon nanotubes are cylindrical molecules that consist of rolled-up sheets of single-layer carbon atoms (graphene); they possess unique properties like high aspect ratio, mechanical strength, electrical and thermal conductivity, chemical stability, and a tip-surface area near the theoretical limit. They are one of the strongest materials known to man.
Category: chemistry – Page 188

Planetary Ingestion Unveiled: Twin Stars Devouring Planets Revealed
Dr. Fan Liu: “Thanks to this very high precision analysis, we can see chemical differences between the twins. This provides very strong evidence that one of the stars has swallowed planets or planetary material and changed its composition.”
Can stars eat planets? This is what a recent study published in Nature hopes to address as a team of international researchers led by ASTRO 3D researchers investigated how some pairs of twin stars possess different compositions, which contradicts longstanding theories that they should possess similar compositions, hence the same twin stars. However, astronomers now hypothesize the compositional differences could be due to one of the twin stars devouring planets that orbit them. This study holds the potential to help astronomers better understand the formation and evolution of planetary systems and the mechanisms behind them, as well.
For the study, the team used a combination of the 6.5-meter Magellan Telescope, the European Southern Observatory’s Very Large Telescope, and the 10-meter Keck Telescope to collect data on 91 twin stars to ascertain their chemical compositions, and specifically the similarity of their compositions. In the end, the team discovered that approximately eight percent (7−8 twin stars) exhibited differences in their compositions, with the team hypothesizing that this was due to one of the stars ingesting one of their orbiting planets. Additionally, they found that the differing pairs were all main sequence stars, meaning they’re average-aged and conducting their fusion at their full potential. For context, our Sun is a main sequence star.

Emergence of an orphan nitrogenase protein following atmospheric oxygenation
Researchers report the birth of a ~2-billion-year-old orphan gene following #planetary #oxygenation, and how this humble beginning shaped the global planetary #ecosystem.
From so simple, a beginning: https://oup.silverchair-cdn.com/UI/app/svg/i.svg?versionId=192134
Abstract. Molecular innovations within key metabolisms can have profound impacts on element cycling and ecological distribution. Yet, much of the molecular foundations of early evolved enzymes and metabolisms are unknown. Here, we bring one such mystery to relief by probing the birth and evolution of the G-subunit protein, an integral component of certain members of the nitrogenase family, the only enzymes capable of biological nitrogen fixation. The G-subunit is a Paleoproterozoic-age orphan protein that appears more than 1 billion years after the origin of nitrogenases. We show that the G-subunit arose with novel nitrogenase metal dependence and the ecological expansion of nitrogen-fixing microbes following the transition in enviromental metal availabilities and atmospheric oxygenation that began ∼2.5 billion years ago. We identify molecular features that suggest early G-subunit proteins mediated cofactor or protein interactions required for novel metal dependency, priming ancient nitrogenases and their hosts to exploit these newly diversified geochemical environments. We further examined the degree of functional specialization in G-subunit evolution with extant and ancestral homologs using laboratory reconstruction experiments. Our results indicate that permanent recruitment of the orphan protein depended on the prior establishment of conserved molecular features and showcase how contingent evolutionary novelties might shape ecologically important microbial innovations.

Scientists Working on Pill You Can Take Instead of Exercising
The future is going to be so lazy, yet so cut.
As next-generation weight-loss treatments like Wegovy and Zepbound continue to fly off the shelves, scientists are busy working on a medicine that could mimic the effects of exercise.
As explained in an American Chemical Society press release, trials thus far on SLU-PP-332, the potentially groundbreaking compound in question, show that it seems “capable of mimicking the physical boost of working out.”
“We cannot replace exercise; exercise is important on all levels,” Bahaa Elgendy, an anesthesiology professor at Washington University Medical School in St. Louis who serves as the principal investigator of the new compound, said in the press release. “If I can exercise, I should go ahead and get the physical activity. But there are so many cases in which a substitute is needed.”

New technique converts excess renewable energy to natural gas
Four Lawrence Livermore National Laboratory (LLNL) researchers have partnered with Los Angeles-based SoCalGas and Munich, Germany-based Electrochaea to develop an electrobioreactor to allow excess renewable electricity from wind and solar sources to be stored in chemical bonds as renewable natural gas.
When renewable electricity supply exceeds demand, electric-utility operators intentionally curtail production of renewable electricity to avoid overloading the grid. In 2020, in California, more than 1.5 million megawatt hours of renewable electricity were curtailed, enough to power more than 100,000 households for a full year.
This practice also occurs in other countries. The team’s electrobioreactor uses the renewable electricity to convert water into hydrogen and oxygen. The microbes then use the hydrogen to convert carbon dioxide into methane, which is a major component of natural gas. Methane can then be moved around in natural gas pipelines and can be stored indefinitely, allowing the renewable energy to be recovered when it is most needed.
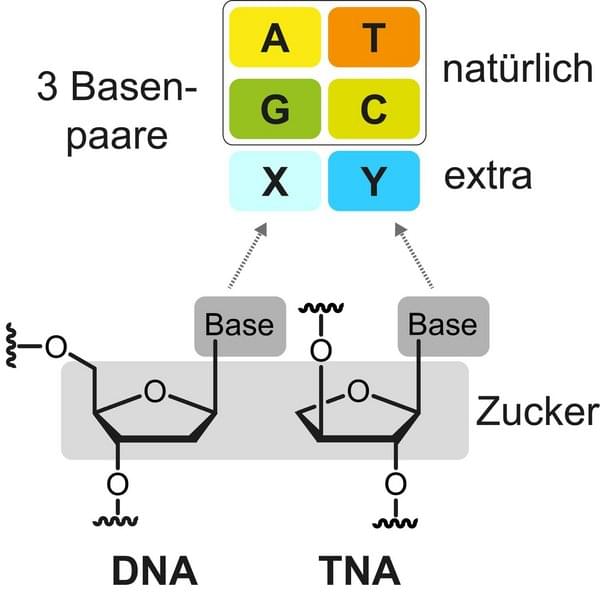
Researchers Develop Artificial Building Blocks of Life
For the first time, scientists have developed artificial nucleotides, the building blocks of DNA, with several additional properties in the laboratory. The DNA carries the genetic information of all living organisms and consists of only four different building blocks, the nucleotides. Nucleotides are composed of three distinctive parts: a sugar molecule, a phosphate group and one of the four nucleobases adenine, thymine, guanine and cytosine. The nucleotides are lined up millions of times and form the DNA double helix, similar to a spiral staircase. Scientists from the UoC’s Department of Chemistry have now shown that the structure of nucleotides can be modified to a great extent in the laboratory.
The researchers developed so-called threofuranosyl nucleic acid (TNA) with a new, additional base pair. These are the first steps on the way to fully artificial nucleic acids with enhanced chemical functionalities. The study ‘Expanding the Horizon of the Xeno Nucleic Acid Space: Threose Nucleic Acids with Increased Information Storage’ was published in the Journal of the American Chemical Society.
Artificial nucleic acids differ in structure from their originals.
Unspoken Triggers of Porn Addiction: Loneliness, Mental Health, and Brain Chemistry
Are you or someone you know struggling with porn addiction? Do you wonder how to quit porn effectively? To quit porn addiction, we need to understand the root causes of porn addiction first. This is a tough subject to talk about, which is why we made this video.

Cannabis Vaping Liquids Contain Nano-Sized Toxic Metal Particles, Study Finds
“Cannabis vapes are newly regulated products in Canada, so we don’t yet have much scientific data about them,” said Dr. Andrew Waye. “This is an opportunity for us to look at some of the questions concerning the risks and unknowns of cannabis vapes.”
Do vapes pose health risks on par with the very tobacco and cannabis products it’s using to safeguard against? This is what a recent study presented at the ACS (American Chemical Society) Spring 2024 meeting hopes to address as a team of researchers investigated the potential health risks that vaping devices could pose, specifically pertaining to the vaping liquids that possess toxic metal nanoparticles, with both regulated and unregulated vaping devices. This study holds the potential to help researchers, medical professionals, and the public better understand the long-term health risks by vaping, which until now have been deemed a “safer” alternative to smoking cigarettes or cannabis.

China is building a railgun that can hurl crewed spacecraft into orbit
And the g-forces???
Rockets being passé, China is working on using an electromagnetic railgun to launch crewed spacecraft the size of a Boeing 737, weighing 50 tonnes, into orbit. This remarkably ambitious project is even more ambitious than it seems at first glance.
Call it a railgun, a catapult, or a mass driver, the idea of replacing rockets with an electromagnetic accelerator is a very attractive option. Instead of lifting off on chemical rockets that have to carry fuel and fuel to lift the fuel and fuel to lift the fuel and the additional fuel, it makes more sense to keep as much of the launching system on the ground while leaving the vehicle as light as possible.
The principle behind such a space railgun is simple, but the details are surprisingly complex and the numbers involved very quickly become daunting. If China can carry off using such a system to launch a spaceplane as part of its Tengyun project that began in 2016, it would be one of history’s major engineering achievements.