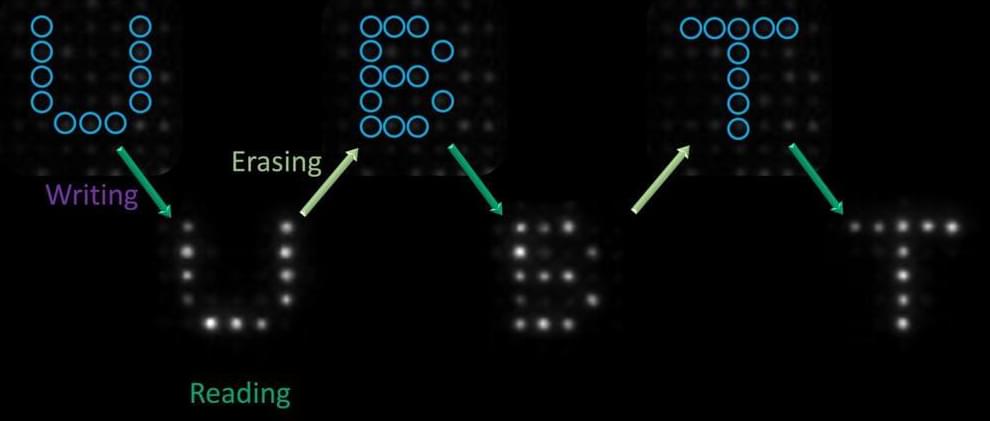
The observation of quantum modifications to a well-known chemical law could lead to performance improvements for quantum information storage.
The Arrhenius law says that the rate of a chemical reaction should decrease steadily as you increase the energy barrier between initial and final states. Now researchers have found a system that obeys a quantum version of the Arrhenius law, where the rate does not drop smoothly but instead decreases in a staircase pattern [1]. The system is a type of quantum bit (qubit) that is particularly robust against environmental disturbances. The researchers demonstrated that they can take advantage of this quantum effect to improve the qubit’s performance.
Technologies such as quantum computers and quantum cryptography use qubits to store information, and one of the continuing challenges is that uncontrolled environmental effects can change the state of a qubit. The most common solutions require large amounts of hardware, but an alternative method is to use qubits that are more error resistant, such as so-called cat qubits. The information in these qubits is stored in robust combinations of quantum states that resemble the states in Schrödinger’s famous feline thought experiment (see Synopsis: Quantum-ness Put on the Scale).