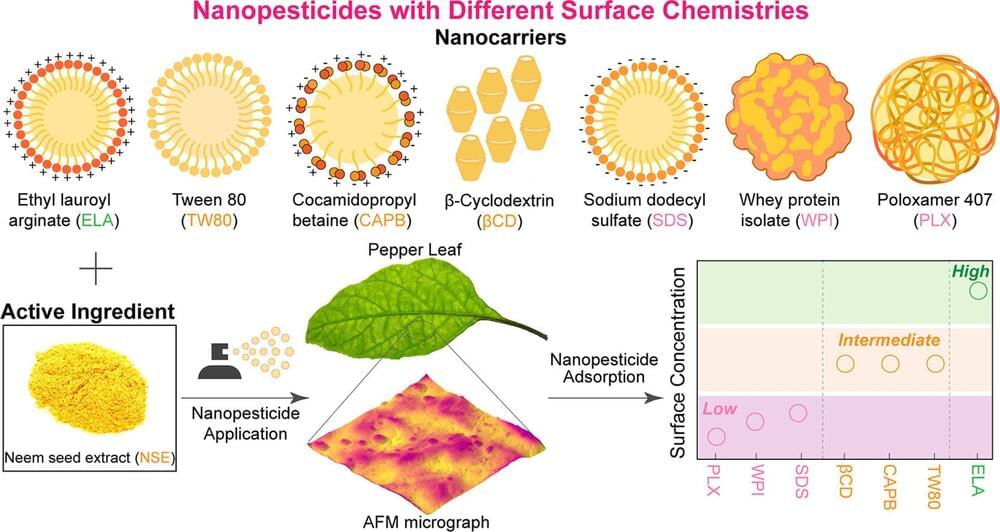
Bacteria modify their ribosomes when exposed to widely used antibiotics, according to research published in Nature Communications. The subtle changes might be enough to alter the binding site of drug targets and constitute a possible new mechanism of antibiotic resistance.
Escherichia coli is a common bacterium which is often harmless but can cause serious infections. The researchers exposed E. coli to streptomycin and kasugamycin, two drugs which treat bacterial infections. Streptomycin has been a staple in treating tuberculosis and other infections since the 1940s, while kasugamycin is less known but crucial in agricultural settings to prevent bacterial diseases in crops.
Both antibiotics tamper with bacteria’s ability to make new proteins by specifically targeting their ribosomes. These molecular structures create proteins and are themselves made of proteins and ribosomal RNA. Ribosomal RNA is often modified with chemical tags that can alter the shape and function of the ribosome. Cells use these tags to fine tune protein production.