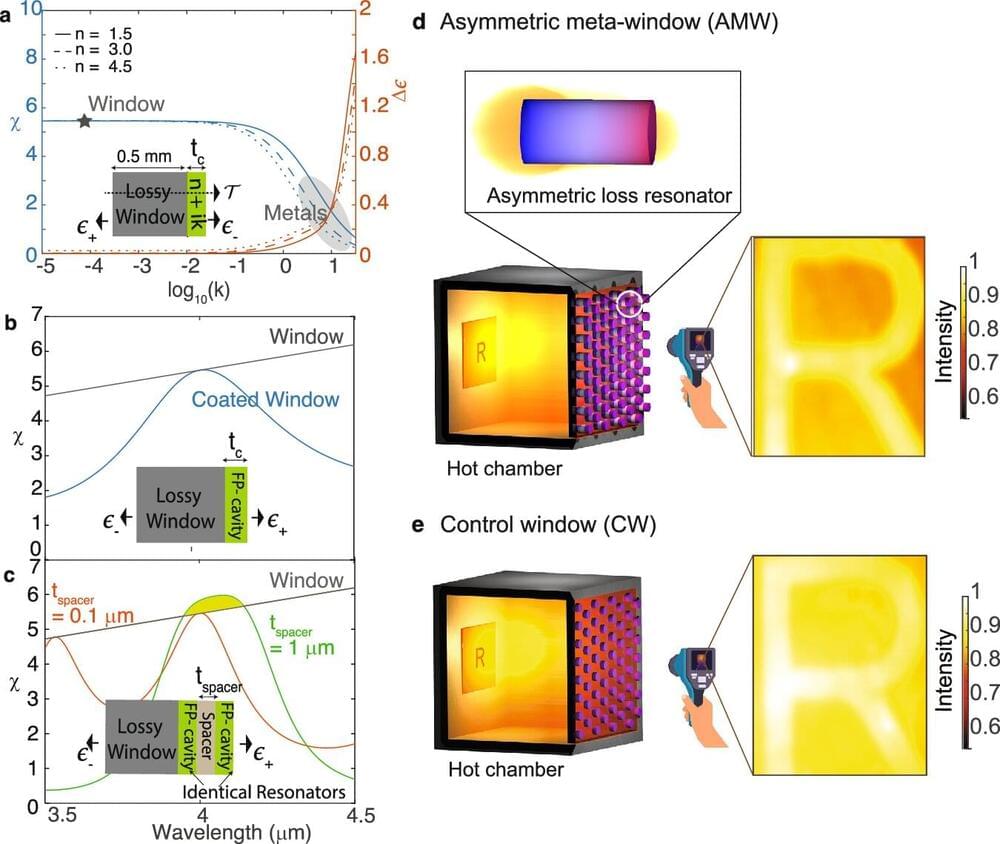
Current laser technologies for the extended short-wave infrared (SWIR) spectral range rely on expensive and complex materials, limiting their scalability and affordability. To address these challenges, ICFO researchers have presented a novel approach based on colloidal quantum dots in an Advanced Materials article. The team managed to emit coherent light (a necessary condition to create lasers) in the extended SWIR range with large colloidal quantum dots made of lead sulfide (PbS).
This new CQD-based technology offers a solution to the aforementioned challenges while maintaining compatibility with silicon CMOS platforms (the technology used for constructing integrated circuit chips) for on-chip integration.
Their PbS colloidal quantum dots are the first semiconductor lasing material to cover such a broad wavelength range. Remarkably, the researchers accomplished this without altering the dots’ chemical composition. These results pave the way towards the realization of more practical and compact colloidal quantum dots lasers.