Category: biotech/medical – Page 825

New personalised cancer vaccine could soon be made available
Hailed as a “true breakthrough”, a new personalised cancer vaccine could soon be made available to patients. Today medical expert Dr Nick Coatsworth explains how the treatment works. Subscribe and 🔔: http://9Soci.al/KM6e50GjSK9 | Get more breaking news at 9News.com.au: http://9Soci.al/iyCO50GjSK6
FOLLOW 9News Australia.
► Facebook: https://www.facebook.com/9News/
► Twitter: https://twitter.com/9NewsAUS
► Instagram: https://www.instagram.com/9news/
Join 9News for the latest in news and events that affect you in your local city, as well as news from across Australia and the world.
#9News #BreakingNews #NineNewsAustralia #9NewsAUS

Genetic Factors that Could Extend the Life of Golden Retrievers
One of the most popular dog breeds is the Golden Retriever. Unfortunately, these dogs are also at high risk for developing cancer. New research has investigated genetic factors that may be able to extend the lives of these beloved dogs. This work focused on longevity genes instead of those that have been associated with cancer, and led to the identification of gene variants that could extend the dogs’ lifespan by as much as two years. The findings have been reported in GeroScience.
While most golden retrievers are predisposed to cancer, some of these dogs can live to be as old as 15 or 16 years. So the researchers thought that there might be genetic factors that were mitigating the effect of the cancer-related genes, noted co-corresponding study author Robert Rebhun, Maxine Adler Endowed Chair in oncology at the UC Davis School of Veterinary Medicine. The gene that had this effect was HER4.
Scientists record powerful signal in the brain’s white matter
The human brain is made up of two kinds of matter: the nerve cell bodies (gray matter), which process sensation, control voluntary movement, and enable speech, learning and cognition, and the axons (white matter), which connect cells to each other and project to the rest of the body.
Historically, scientists have concentrated on the gray matter of the cortex, figuring that’s where the action is, while ignoring white matter, even though it makes up half the brain. Researchers at Vanderbilt University are out to change that.
For several years, John Gore, Ph.D., director of the Vanderbilt University Institute of Imaging Science, and his colleagues have used functional magnetic resonance imaging (fMRI) to detect blood oxygenation-level dependent (BOLD) signals, a key marker of brain activity, in white matter.
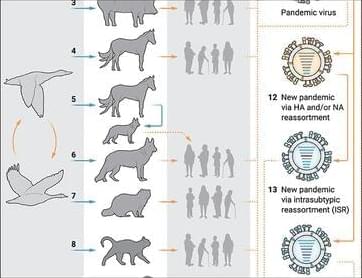
Many potential pathways to future pandemic influenza
Influenza viruses are believed to have sparked at least 14 human pandemics in the past 500 years; the most devastating of which began in 1918. Yet, despite intense study and considerable advances in public health, virus surveillance and virology, there is no simple answer to this pressing question: when and how will the next flu pandemic arise?
NIAID scientists including Jeffery K. Taubenberger, M.D., Ph.D., consider the many potential pathways to future influenza pandemics in a new viewpoints essay in Science Translational Medicine. There are no hard and fast ‘rules’ specifying, for example, what characteristics a given avian influenza virus must possess to allow it to efficiently infect… More.
Influenza pandemics have emerged for centuries but still cannot be accurately predicted.
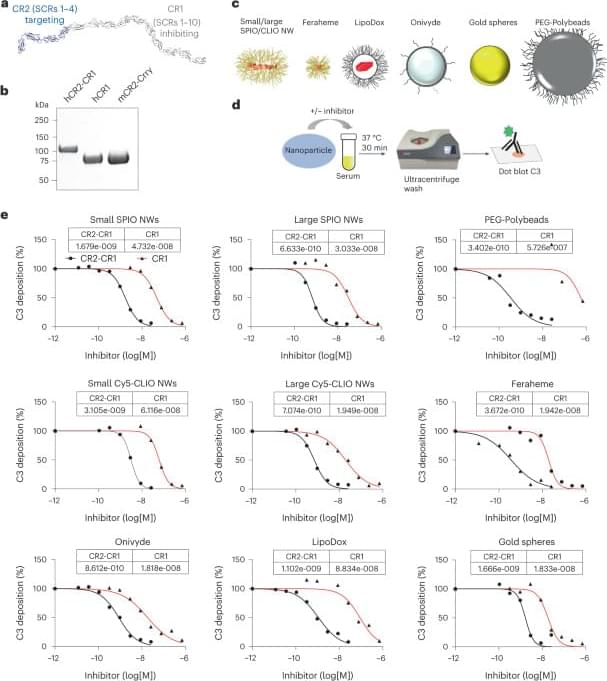
Inhibition of acute complement responses towards bolus-injected nanoparticles using targeted short-circulating regulatory proteins
A NIAID-funded study suggests a strategy to mitigate harmful side effects of nanoparticles in medicine. The researchers showed in animal models that a lab-made molecule safely prevented nanomedicines from activating a set of immune-system proteins called the complement system and causing negative side effects. This is significant because when nanoparticles activate complement, the resulting immune response can not only cause an adverse reaction, but also reduce the efficacy of nanomedicines.

Breast Health: Follow-up after an abnormal mammogram
Breast changes are very common, and most breast changes are not cancer. Our health guide for women explains:
A breast lump may be benign or a symptom of breast cancer. Learn about follow-up after an abnormal mammogram. See pictures of breast cancer, cysts, and calcifications. Find out symptoms for benign breast conditions, precancers, and DCIS.
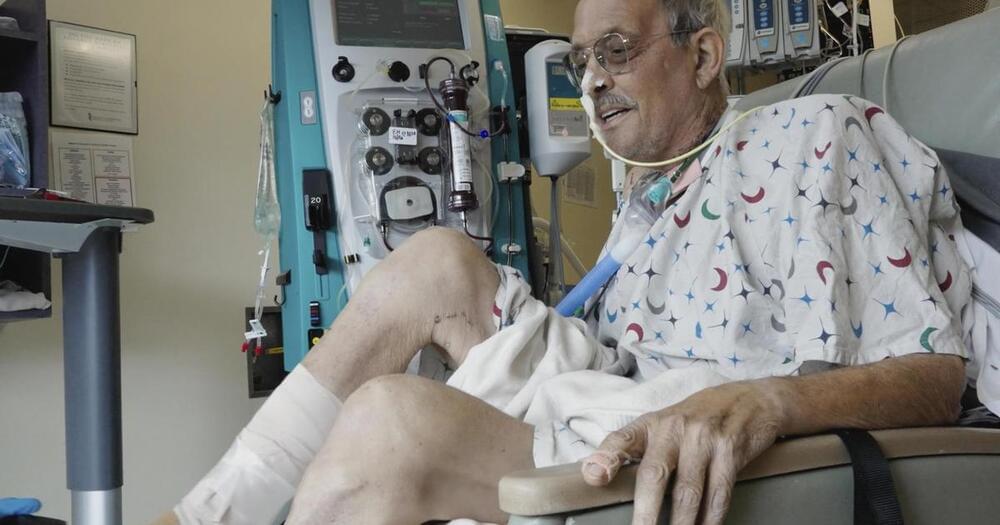
Month after pig heart transplant, Maryland man pushing through “tough” physical therapy
It’s been a month since a Maryland man became the second person to receive a transplanted heart from a pig — and hospital video released Friday shows he’s working hard to recover.
Lawrence Faucette was dying from heart failure and ineligible for a traditional heart transplant when doctors at the University of Maryland School of Medicine offered the highly experimental surgery.
In the first glimpse of Faucette provided since the Sept. 20 transplant, hospital video shows physical therapist Chris Wells urging him to push through a pedaling exercise to regain his strength.