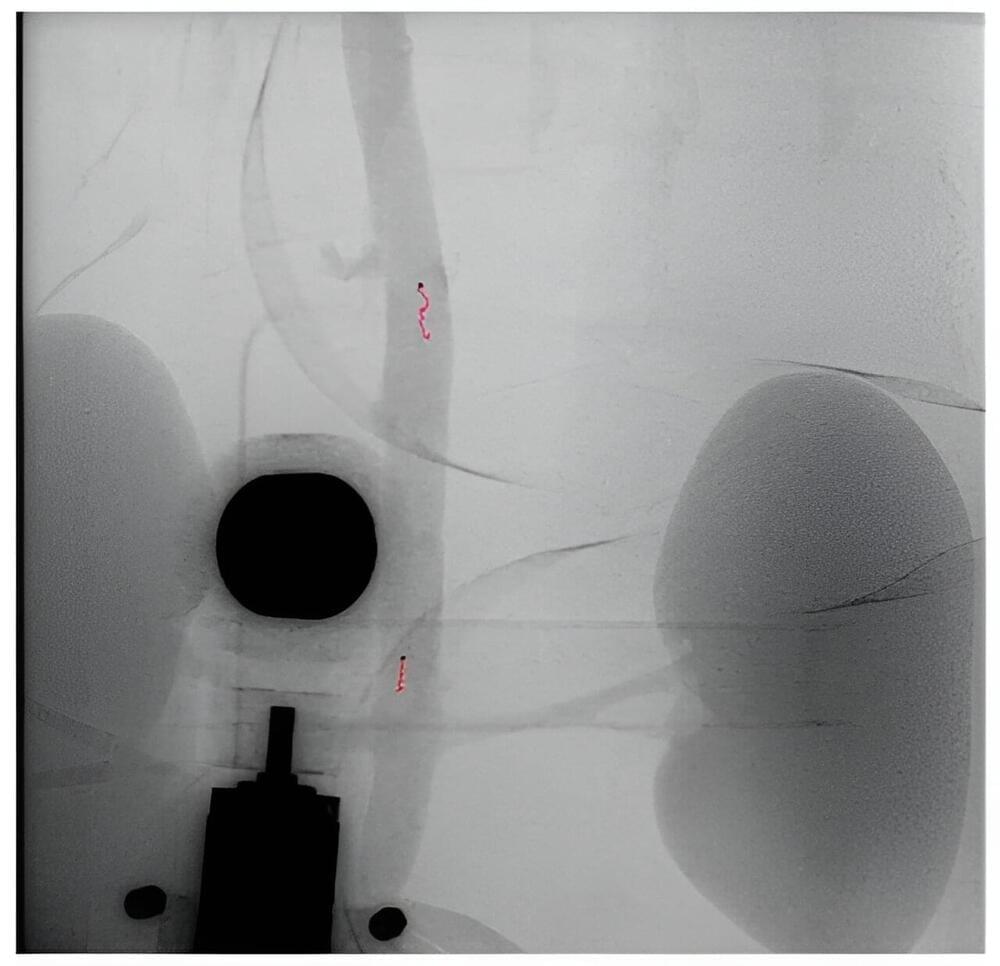
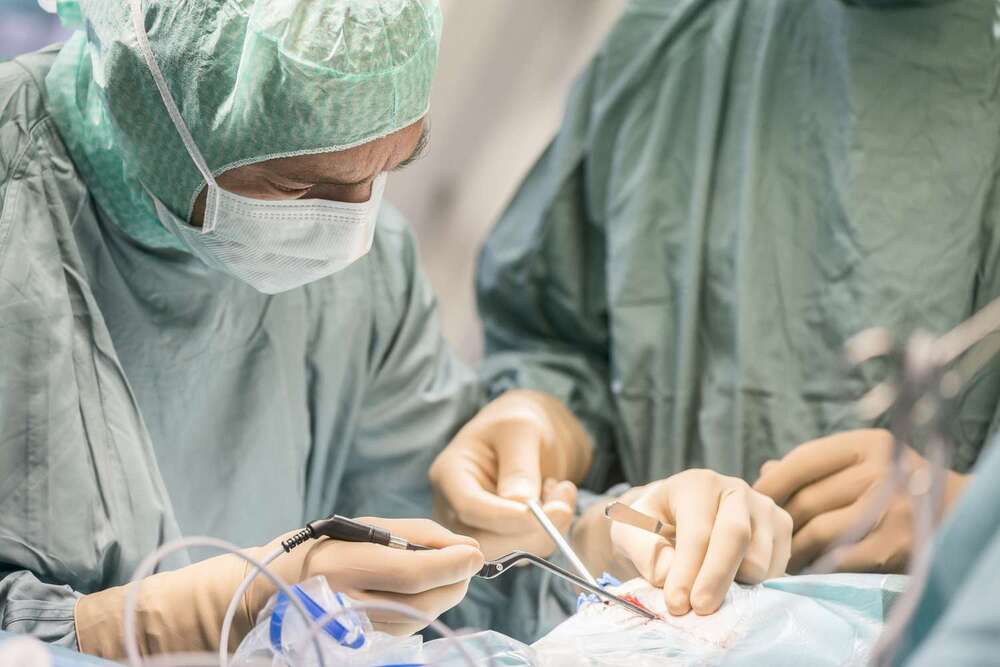
For the first time ever, wireless millirobots navigated a narrow blood vessel both along and against arterial flow. Researchers from the University of Twente and Radboudumc inserted the screw-shaped robots in a detached aorta with kidneys where they controlled them using a robotically controlled rotating magnet. The researchers plan to further develop the technology to be able to remove blood clots.
Each year worldwide, one in four people die from conditions caused by blood clots. A blood clot blocks a blood vessel preventing the blood from delivering oxygen to certain areas of the body. Surgeons can use flexible instruments to remove the blood clot therefore allowing the blood to flow again, but some regions in the body are difficult to reach. Millirobots can overcome these limitations and remove blood clots from difficult-to-reach blood vessels.
The researchers showed that these millirobots were able to travel through blood vessels. But to do so, the millirobots need power, to travel up-and downstream and to accurately be controlled and localized. Last but not least, they need to be biocompatible and leave no further damage to the inside of blood vessels.