Vaccines and antivirals are already undergoing trials.


Inside her small office, with a window overlooking the iconic Kerckhoff Hall student centre at University of California, Los Angeles (UCLA), chemical biologist Mireille Kamariza is pursuing her big dream. Since 2015, she has steadily worked to stop transmission of deadly tuberculosis (TB) superbugs, which in 2022 infected more than 10 million people and killed more than one million.
As a PhD student working with Nobel laureate Carolyn Bertozzi, now a chemist at Stanford University in California, she developed a fluorescent diagnostic test that could be used for quick detection of TB, especially in resource-poor settings. In 2019, alongside Bertozzi, Kamariza founded the biotech start-up company OliLux Biosciences, based in Los Angeles, to develop reliable tools for detecting TB that are tailored to the complex needs of poorer countries. Nature sat down with Kamariza to talk about her progress in testing these diagnostic tools for use in the real world, and the uphill battle in fighting the spread of TB.
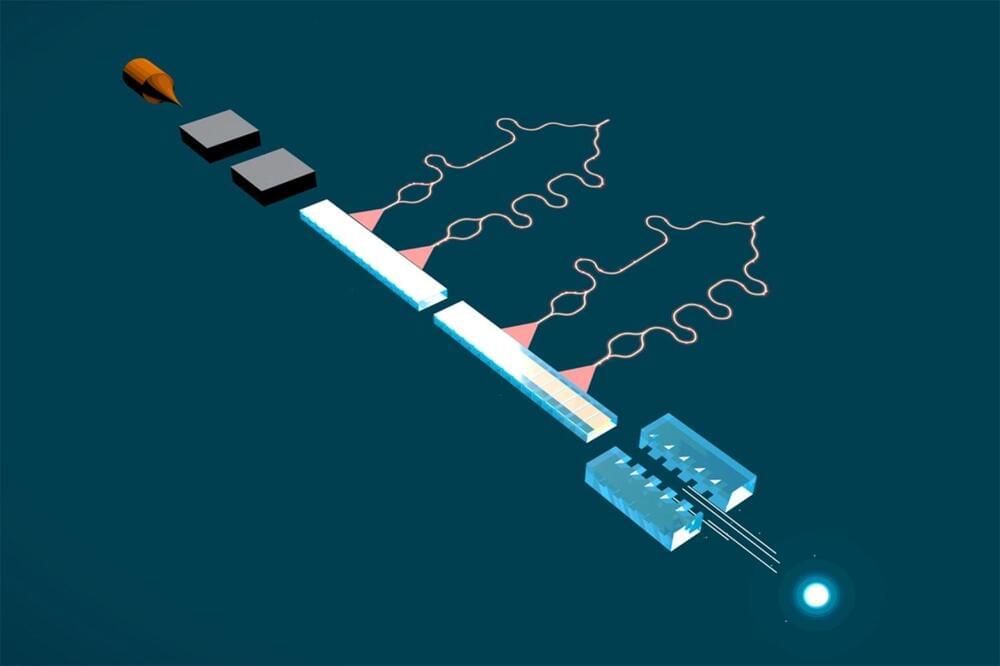
A new advance by Stanford engineers could lead to particle accelerators being widely available in science, medicine, and industry.
Stanford researchers are getting closer to building a tiny electron accelerator based on “accelerator-on-a-chip” technology with broad potential applications in studying physics as well as medical and industrial uses.
The researchers have demonstrated that a silicon dielectric laser accelerator, or DLA, can now both speed up and confine electrons, creating a focused beam of high-energy electrons. “If the electrons were microscopic cars, it’s as if, for the first time, we’re steering and we have our foot on the gas,” said Payton Broaddus, PhD ’23 in electrical engineering and the lead author on a paper published on February 23 detailing the breakthrough in Physical Review Letters.
The pursuit of a cure for Alzheimer’s disease is becoming an increasingly competitive and contentious quest with recent years witnessing several important controversies.
In July 2022, Science magazine reported that a key 2006 research paper, published in the prestigious journal Nature, which identified a subtype of brain protein called beta-amyloid as the cause of Alzheimer’s, may have been based on fabricated data.
One year earlier, in June 2021, the US Food and Drug Administration had approved aducanumab, an antibody-targeting beta-amyloid, as a treatment for Alzheimer’s, even though the data supporting its use were incomplete and contradictory.

“Cannabis use is increasing in both prevalence and frequency, while conventional tobacco smoking is declining,” said Salomeh Keyhani, MD, MPH. “Cannabis use by itself might, over time, become the more important risk factor.”
Can smoking cannabis bring the same risk of heart attack and stroke as smoking cigarettes? This is what a recent study published in the Journal of the American Heart Association hopes to address as a team of researchers from the University of California, San Francisco (UCSF) investigated the likelihood that cannabis use would lead to a heart attack and/or stroke. This study comes as recreational cannabis use is slowly becoming legal across the United States and holds the potential to help researchers, medical professionals, legislators, and the public better understand the long-term health risks associated with cannabis use, specifically smoking cannabis.
For the study, the team compared data from the Behavioral Risk Factor Surveillance Survey between 2016 and 2020 across 27 American states and 2 territories and 434,104 survey participants between ages 18 and 74 to ascertain a link between their cannabis use and likelihood for heart problems. The team classified cannabis use as the number of times a participant smoked cannabis within a previous 30 days while accounting for self-reported heart issues and tobacco use, as well.
In the end, the team found that 4 percent of participants use cannabis daily while 7.1 percent did not. When combined with the self-reported heart issues of the daily cannabis users, the team found this group exhibited a 25 percent chance of having a heart attack and a 42 percent chance of having a stroke.

Researchers in Imperial College London’s Department of Materials have developed a new portable maser that can fit the size of a shoebox.
Imperial College London pioneered the discovery of room-temperature solid-state masers in 2012, highlighting their ability to amplify extremely faint electrical signals and demonstrate high-frequency stability. This was a significant discovery because microwave signals can pass through the Earth’s atmosphere more easily than other wavelengths of light. Additionally, microwaves have the capability to penetrate through the human body, a feat not achievable by lasers.
Masers have extensive applications in telecommunications systems—everything from mobile phone networks to satellite navigation systems. They also have a key role in advancing quantum computing and improving medical imaging techniques, like MRI machines. They are typically large, bulky, stationary equipment found only in research laboratories.
A team of Stanford Medicine doctors and biomedical engineers are among the first to integrate a new augmented reality tool into surgical practice. The technology, Apple Vision Pro, is a headset that provides a form of human-computer interaction — it allows its wearer to navigate their surroundings using real-time visual data in combination with virtual elements.
“The novel use of augmented reality in the operating room exemplifies Stanford Medicine’s mission of serving patients in a digitally driven, human-centered care environment,” said Lloyd Minor, dean of the School of Medicine and vice president of medical affairs at Stanford University. “Our health system has long stood at the vanguard for the use of digital technologies in medicine, and I’m proud that through initiatives like RAISE Health, we also define the safe, responsible and equitable use of these innovations.”
A cardiologist used the technology, with the patient’s informed consent, to successfully perform an ablation procedure this week at Stanford Hospital to treat atrial fibrillation.
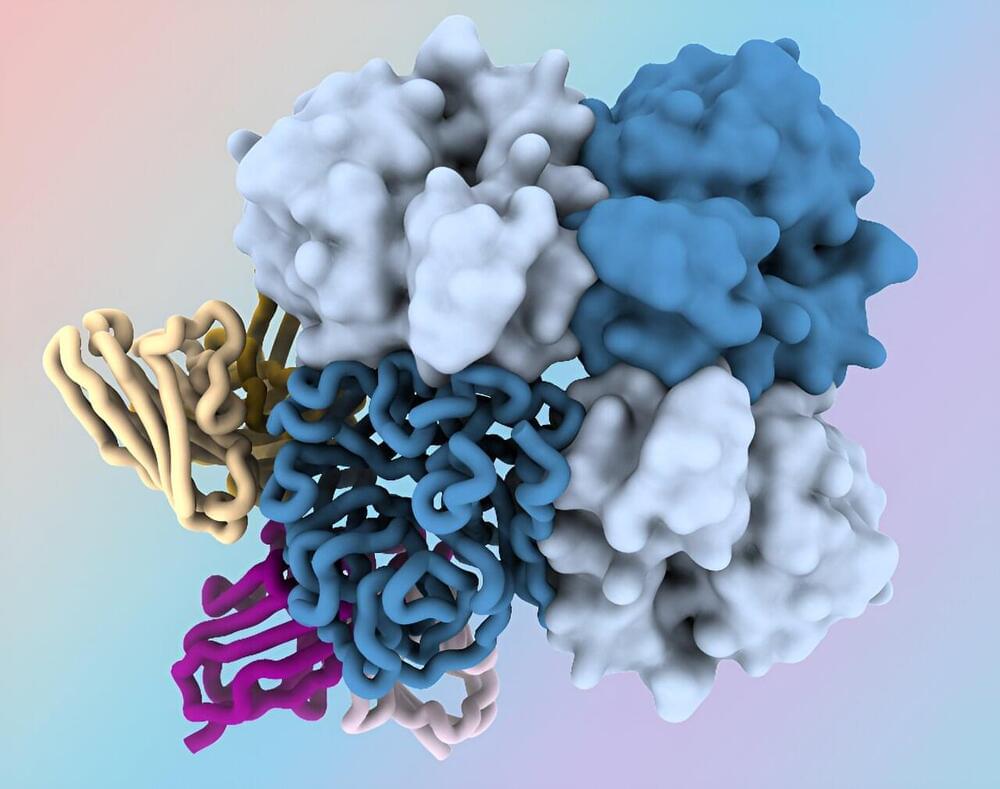
Researchers at the National Institutes of Health have identified antibodies targeting a hard-to-spot region of the influenza virus, shedding light on the relatively unexplored “dark side” of the neuraminidase (NA) protein head. The antibodies target a region of the NA protein that is common among many influenza viruses, including H3N2 subtype viruses, and could be a new target for countermeasures. The research, led by scientists at the National Institute of Allergy and Infectious Diseases’ Vaccine Research Center, part of NIH, was published today in Immunity.
Influenza, or flu, sickens millions of people across the globe each year and can lead to severe illness and death. While vaccination against influenza reduces the burden of the disease, updated vaccines are needed each season to provide protection against the many strains and subtypes of the rapidly evolving virus. Vaccines that provide protection against a broad range of influenza viruses could prevent outbreaks of new and reemerging flu viruses without the need for yearly vaccine reformulation or vaccinations.
One way to improve influenza vaccines and other countermeasures is to identify new targets on the virus’s surface proteins in “conserved” regions—portions that tend to be relatively unchanged between different strains of the virus. Influenza NA is a surface protein containing a globular head portion and a narrow stalk portion.
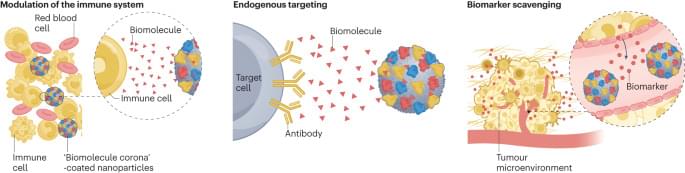
Nanoparticles (NPs) administered in the human body will undergo rapid surface modification upon contact with biological fluids driven by their interfacial interaction with a diverse range of biomolecules. Such spontaneous self-assembly and adsorption of proteins and other biomolecules onto the NP surface constitute what is commonly known as the protein or biomolecule corona. This surface biotransformation of the NPs modulates their biological interactions and impact on physiological systems and can influence their overall pharmacological profile. Here, we comment on how the initially considered ‘nuisance’ of the in vivo corona formation can now be considered a nanoparticle engineering tool for biomedical use, such as in endogenous tissue targeting, personalized biomarker discovery and immunomodulation.

Interestingly enough, although Elon Musk’s Neuralink received a great deal of media attention, early in 2023, Synchron published results from its first-in-human study of four patients with severe paralysis who received its first-generation Stentrode neuroprosthesis implant. The implant allowed participants to create digital switches that controlled daily tasks like sending texts and emails, partaking in online banking, and communicating care needs. The study’s findings were published in a paper in JAMA Neurology in January 2023. Then, before September, the first six US patients had the Synchron BCI implanted. The study’s findings are expected by late 2024.
Let’s return to Upgrade. “One part The Six Million Dollar Man, one part Death Wish revenge fantasy” was how critics described the movie. While Death Wish is a 1974 American vigilante action-thriller movie that is partially based on Brian Garfield’s 1972 novel of the same name, the American sci-fi television series The Six Million Dollar Man from the 1970s, based on Martin Caidin’s 1972 novel Cyborg, could be considered a landmark in the context of human-AI symbiosis, although in fantasy’s domain. Oscar Goldman’s opening line in The Six Million Dollar Man was, “Gentlemen, we can rebuild him. We have the technology. We have the capability to make the world’s first bionic man… Better than he was before. Better—stronger—faster.” The term “cyborg” is a portmanteau of the words “cybernetic” and “organism,” which was coined in 1960 by two scientists, Manfred Clynes and Nathan S Kline.
At the moment, “cyborg” doesn’t seem to be a narrative of a distant future, though. Rather, it’s very much a story of today. We are just inches away from becoming cyborgs, perhaps, thanks to the brain chip implants, although Elon Musk perceives that “we are already a cyborg to some degree,” and he may be right. Cyborgs, however, pose a threat, while the dystopian idea of being ruled by Big Brother also haunts. Around the world, chip implants have already sparked heated discussions on a variety of topics, including privacy, the law, technology, medicine, security, politics, and religion. USA Today published a piece headlined “You will get chipped—eventually” as early as August 2017. And an article published in The Atlantic in September 2018 discussed how (not only brain chips but) microchip implants, in general, are evolving from a tech-geek curiosity to a legitimate health utility and that there may not be as many reasons to say “no.” But numerous concerns about privacy and cybersecurity would keep us haunted. It would be extremely difficult for policymakers to formulate laws pertaining to such sensitive yet quickly developing technology.