🩸📉🧬
Research found that younger age at type 2 diabetes diagnosis is associated with higher risks of cardiovascular and all-cause mortality, emphasizing the need for early prevention and personalized management strategies.

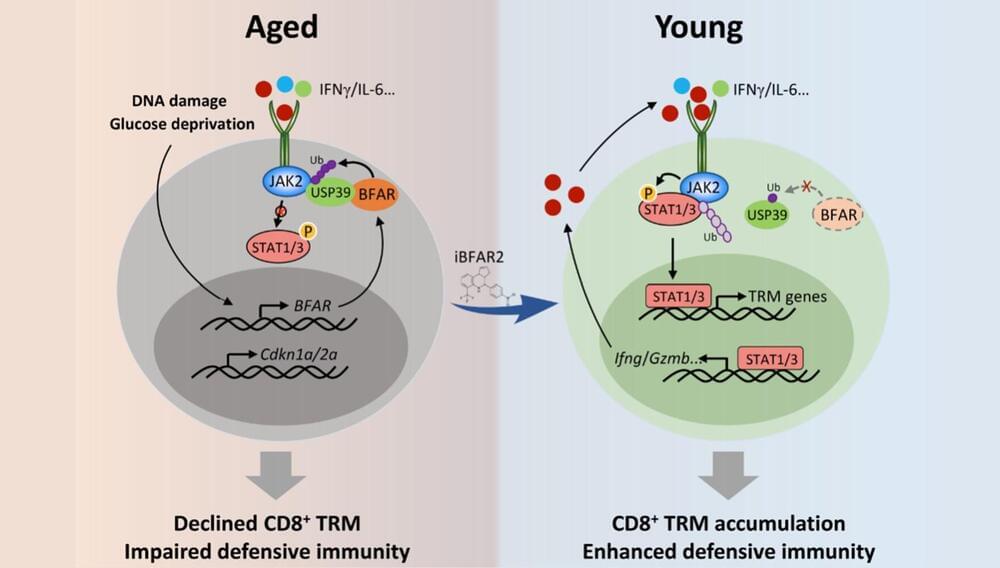
A research team from the Shanghai Institute of Nutrition and Health (SINH) of the Chinese Academy of Sciences has revealed that aging specifically impairs the generation of CD8+ tissue resident memory T cells (TRM) and thus compromises the antitumor defensive activity of aged CD8+ T cells. The study is published in Nature Aging.
With the aging process, the risk of developing cancer significantly increases. In recent years, it has been reported that immune aging has an important impact on tumor development. Immune aging is a degenerative change in the immune system that occurs with aging, leading to a decline in immune response and ultimately triggering diseases including tumors.
Within the immune system, CD8+ T cells are the main defensive adaptive immune cells protecting against tumor cells. However, the mechanism by which aging impairs the antitumor response of CD8+ T cells was not previously understood.

An injection given during some asthma and COPD attacks is more effective than the current treatment of steroid tablets, reducing the need for further treatment by 30%. The findings, published in The Lancet Respiratory Medicine, could be “game-changing” for millions of people with asthma and COPD around the world, scientists say.
Asthma attacks and COPD flare-ups (also called exacerbations) can be deadly. Every day in the UK four people with asthma and 85 people with COPD will tragically die. Both conditions are also very common. In the UK, someone has an asthma attack every 10 seconds. Asthma and COPD cost the NHS £5.9B a year.
The type of symptom flare-up the injection treats are called “eosinophilic exacerbations” and involve symptoms such as wheezing, coughing and chest tightness due to inflammation resulting from high amounts of eosinophils (a type of white blood cell). Eosinophilic exacerbations make up to 30% of COPD flare-ups and almost 50% of asthma attacks. They can become more frequent as the disease progresses, leading to irreversible lung damage in some cases.
To maintain a healthy immune system, doctors advise patients to take vitamins and minerals. Vitamins have many functions that benefit the body, including resisting infection, energy boost, aiding in blood clotting, improving brain function, generation of red blood cells, promoting a healthy gut microbiome, improving wound healing, preventing eye deterioration, and developing strong bones. We can get vitamins from various sources, including orange juice, which is rich in vitamin C, folate, and potassium. Physicians often recommend supplements for patients low on specific vitamins. However, dysregulation of vitamins can weaken the immune system and promote overall bad health. One vitamin in particular that helps maintain cellular function includes B12. This vitamin is essential to generate DNA and red blood cells, and aids in nerve function, energy conversion, and protein metabolism. When a patient has a B12 deficiency it can result in muscle weakness, numbness in hands and feet, difficulty walking, nausea, loss of appetite, and unintentional weight loss. In addition, it can allow the buildup of a small molecule known as methylmalonic acid (MMA).
In healthy tissues, vitamin B12 helps break down MMA. In B12 deficient patients, MMA is increased and can be measured through blood or urine samples. Methylmalonic acid is produced when proteins in your muscle, known as amino acids, are broken down. Tests to determine B12 deficiency or a genetic disorder are done by physicians at birth and after the appearance of symptoms related to B12 deficiency. Interestingly, a group of scientists have discovered a new deleterious role of MMA in lung carcinoma.
A recent publication from Oncogene, by Dr. Ana P. Gomes and others, demonstrated that MMA in aged patients weakens immune cell function and promotes lung cancer progression. Gomes is a professor of molecular oncology at Moffitt Cancer Center in Florida. Her work specifically focuses on understanding metabolic changes as we age and how this change in metabolism influences cancer risk.
Whether it’s our phones, cars, televisions, medical devices or even washing machines, we now have computers everywhere.
Using bigger computers, we solve bigger problems like managing the operation of a power grid, designing an aircraft, predicting the weather or providing different types of artificial intelligence (AI).
But all these machines work by manipulating data in the form of ones and zeros (bits) using classical techniques that have not changed since the abacus was invented in antiquity.

In case you thought science was going to take a day off, researchers have just figured out a way of reversing brain aging – in fruit flies, but still.
They previously did something similar in lab mice, claiming to “reverse and repair” damage done by Alzheimer’s disease. The brain is a fascinating thing: it behaves weirdly after midnight, performs a magical reset while sleeping to “save memories,” and automatically corrects spelling errors even when you don’t see them yourself. Whatever next, health experts?!
When a common type of protein builds up in the brain, it stops cells from getting rid of “unnecessary or dysfunctional components,” i.e., waste.
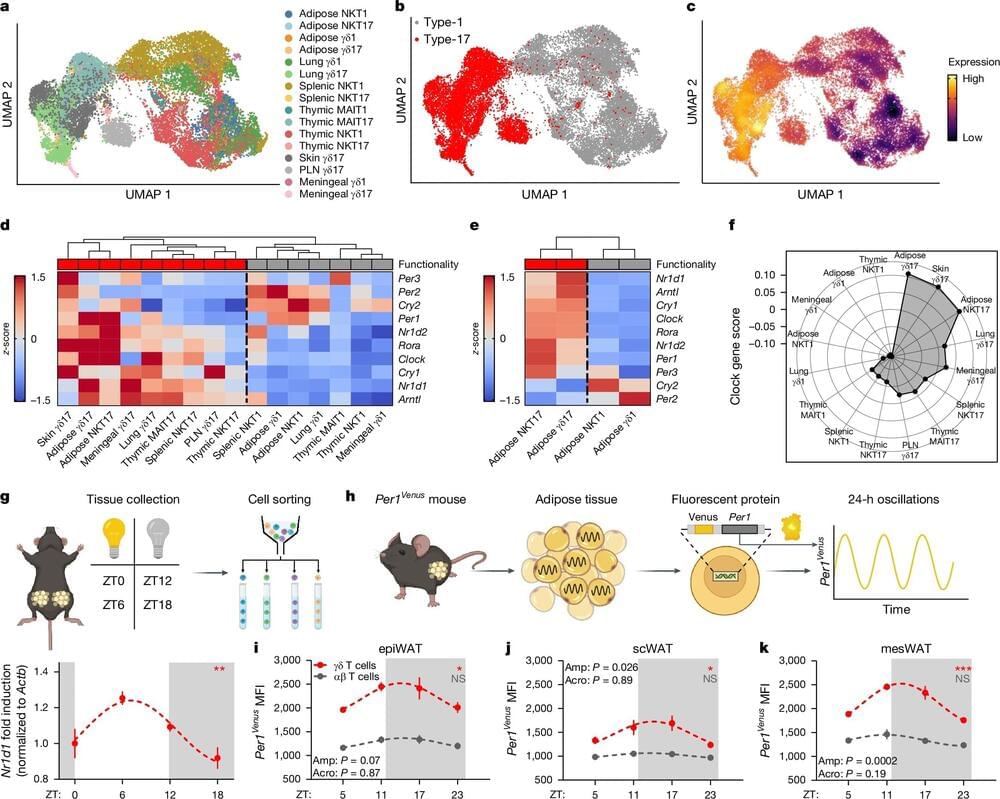
Recent research reveals that the immune system interacts with the body’s internal clock, influencing both fat storage and temperature regulation.
The discovery hints at why shift workers and others with irregular work, eating, or sleep patterns driven by the demands of modern life fall out of metabolic sync, and may hold potential for developing therapies to address obesity and prevent wasting.
The key finding—that an immune molecule within adipose (fat) tissue, known as interleukin-17A (IL-17A), plays a regulatory role in fat storage—holds significant therapeutic potential for addressing obesity, preventing wasting, and mitigating other metabolic disorders. By targeting this molecule, drug developers may gain a valuable new pathway for creating treatments aimed at these conditions.
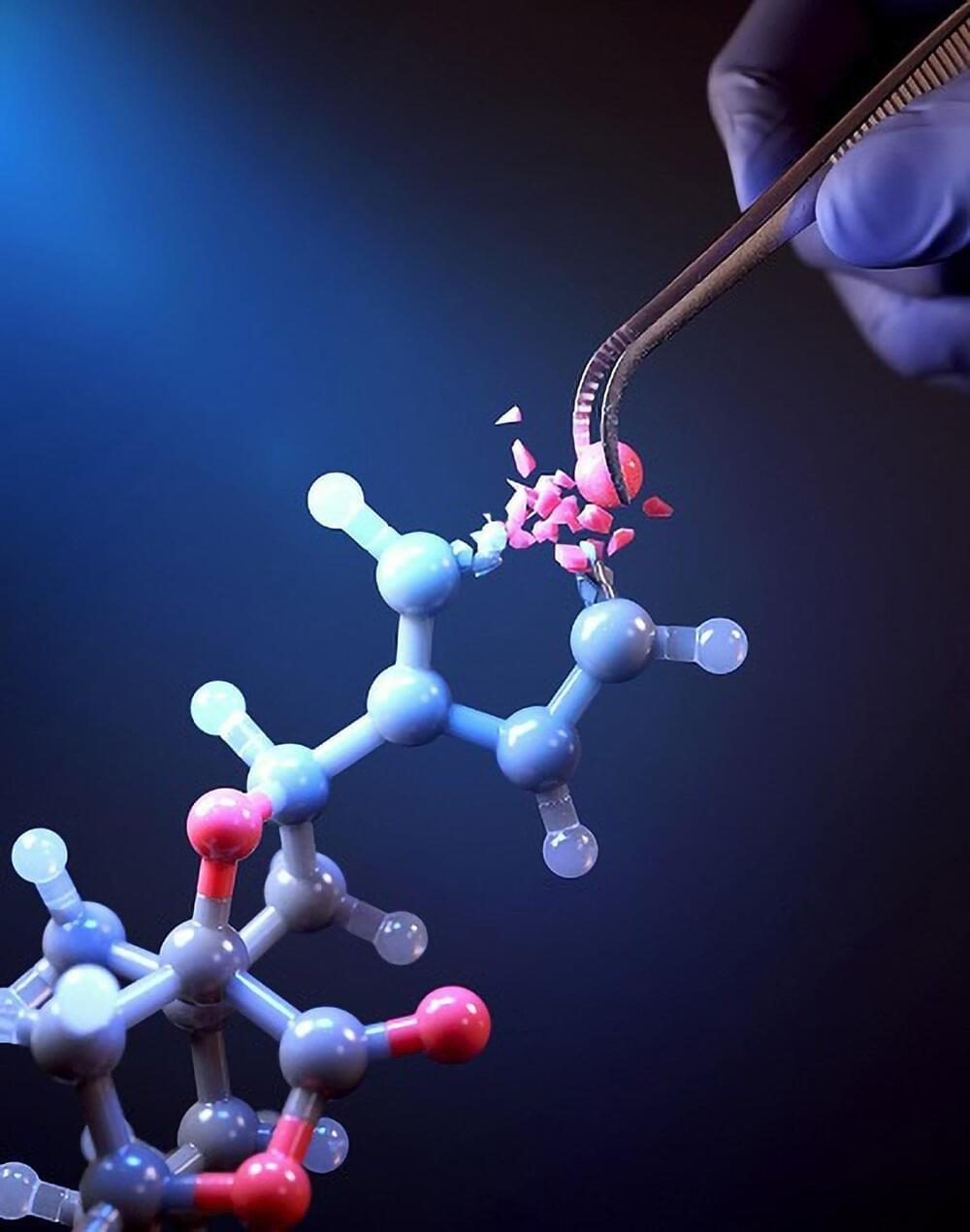
KAIST researchers have developed a groundbreaking single-atom editing technology using light-powered “molecular scissors” to convert oxygen atoms into nitrogen in drug compounds, simplifying drug development and boosting efficacy.
In the field of pioneering drug development, a groundbreaking new technology that enables the precise and rapid editing of key atoms critical to drug efficacy has been hailed as a transformative and “dream” innovation, revolutionizing the process of discovering potential drug candidates. Researchers at KAIST have achieved a world-first by successfully developing single-atom editing technology designed to maximize drug efficacy.
On October 8th, KAIST (represented by President Kwang-Hyung Lee) announced that Professor Yoonsu Park’s research team from the Department of Chemistry successfully developed technology that enables the easy editing and correction of oxygen atoms in furan compounds into nitrogen atoms, directly converting them into pyrrole frameworks, which are widely used in pharmaceuticals.