There are various studies that have explored the role of the body’s circadian rhythm in regulating immune activity. Disruptions in the circadian rhythms exacerbate inflammation. Researchers from the Royal College of Surgeons in Ireland (RCSI) University of Medicine and Health Sciences have previously studied how the immune cells called macrophages are affected without an internal body clock. Now, new research by RCSI describes how macrophages work differently at various times of day and could pave the way for time-targeted treatments for inflammatory diseases. The research also illuminates a key role for mitochondria in driving daily changes in immune activity.
The findings are published in The FASEB Journal in an article titled, “Time-of-day control of mitochondria regulates NLRP3 inflammasome activation in macrophages.”
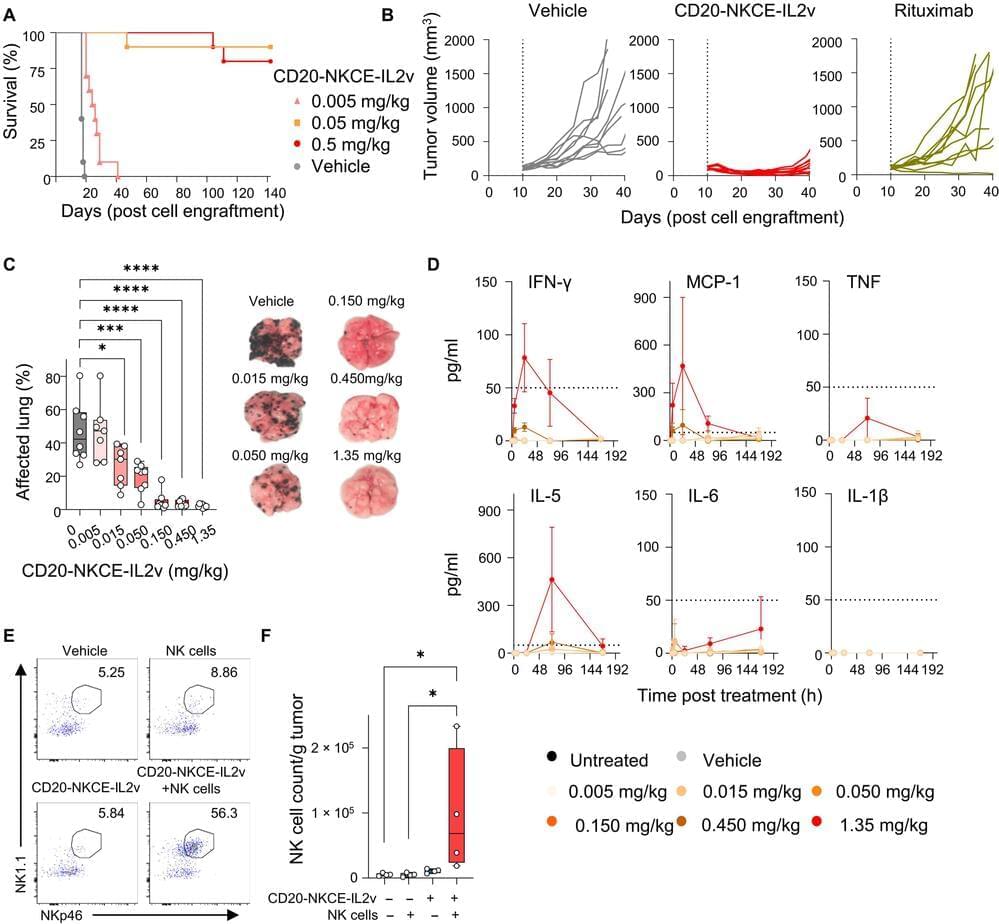
Macrophages release interleukin-1 (IL-1) cytokines in response to inflammatory stimuli, and the NLRP3 inflammasome mediates IL-1-family cytokine release via pyroptosis. Mitochondria play a multifaceted role regulating NLRP3 inflammasome activity. However, whether the macrophage clock regulates the NLRP3 inflammasome via mitochondrial control remains unclear.