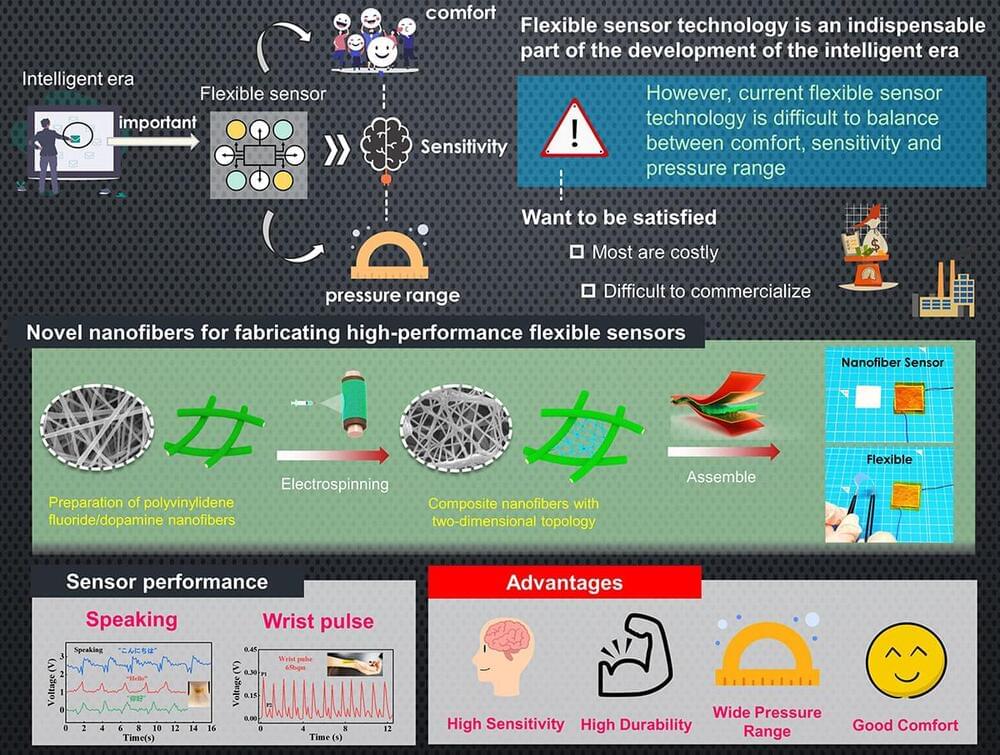
Flexible piezoelectric sensors are essential to monitor the motions of both humans and humanoid robots. However, existing designs are either are costly or have limited sensitivity. In a recent study, researchers from Japan tackled these issues by developing a novel piezoelectric composite material made from electrospun polyvinylidene fluoride nanofibers combined with dopamine. Sensors made from this material showed significant performance and stability improvements at a low cost, promising advancements in medicine, healthcare, and robotics.
The world is accelerating rapidly towards the intelligent era—a stage in history marked by increased automation and interconnectivity by leveraging technologies such as artificial intelligence and robotics. As a sometimes-overlooked foundational requirement in this transformation, sensors represent an essential interface between humans, machines, and their environment.
However, now that robots are becoming more agile and wearable electronics are no longer confined to science fiction, traditional silicon-based sensors won’t make the cut in many applications. Thus, flexible sensors, which provide better comfort and higher versatility, have become a very active area of study. Piezoelectric sensors are particularly important in this regard, as they can convert mechanical stress and stretching into an electrical signal. Despite numerous promising approaches, there remains a lack of environmentally sustainable methods for mass-producing flexible, high-performance piezoelectric sensors at a low cost.